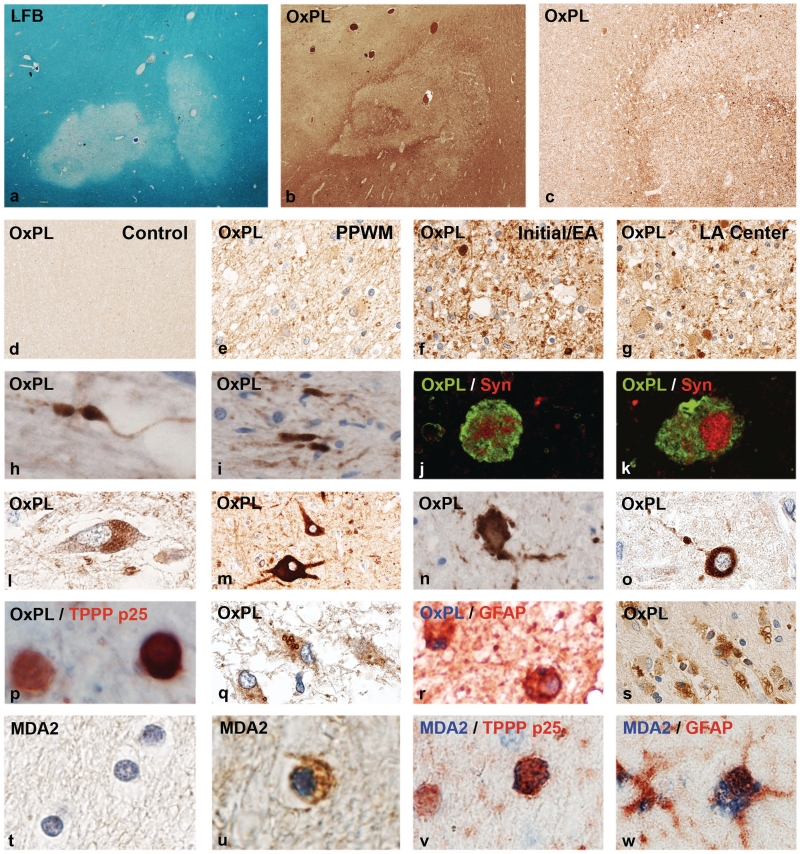
Figure 2.
Oxidized lipids in multiple sclerosis lesions. (a) Myelin loss in an active white matter lesion in acute multiple sclerosis, Marburg’s type (×8). (b) Adjacent serial section from a, stained with the marker E06 for oxidized phospholipids (OxPL) with profound immunoreactivity at the lesion edge and the adjacent peri-plaque white matter (×8). (c) Higher magnification of the sections shown in b, showing increased immunoreactivity for oxidized phospholipids at the lesion edge (×45). (d) Normal white matter of a control patient without neurological disease or CNS lesions, without immunoreactivity for oxidized phospholipids (×45). (e) Peri-plaque white matter (PPWM) from the area shown in b, with increased immunoreactivity, in comparison with the control white matter, but much lower reactivity as at the active lesion edge. Immunoreactivity is mostly associated with myelin (×150). (f) High immunoreactivity for oxidized phospholipids in myelin sheaths and some globular structures, representing axonal spheroids, are present at the edge of the active lesions shown in b (×150). (g) Inactive lesion centre of the same lesion shown in b, with low immunoreactivity for oxidized phospholipids; intensive immunoreactivity, however, is seen in some axonal spheroids (×150). (h–k) Oxidized phospholipids are selectively accumulated within dystrophic axons and axonal spheroids (h, i); the nature of dystrophic axons is documented by double staining confocal microscopy for oxidized phospholipids and synaptophysin (Syn: j and k) (h, i: ×900; j, k: ×2500). (l–o) Accumulation of oxidized phospholipids in neurons in patients with multiple sclerosis with active lesions: (l) lipofuscin reactivity in cortical neuron; (m) ballooned cortical neuron adjacent to destructive subcortical lesion with intense immunoreactivity for oxidized phospholipids; (n, o) neurons with intense cytoplasmic reactivity for oxidized phospholipids with fragmented cell processes (dendrites) in active lesions in the basal ganglia (h) and cortex (o) (×600). (p) Active multiple sclerosis lesions; double staining for the oligodendrocyte marker TPPP-p25 (red) and (black/brown), showing one of the two oligodendrocytes with intense cytoplasmic E06 immunoreactivity (×900). (q, r) In astrocytes, oxidized phospholipids are sequestered in the cytoplasm in the form of larger granules, possibly representing autophagic vacuoles (q: ×450; r: ×750). (s) Macrophages with granular cytoplasmic reactivity for oxidized phospholipids in the centre of an active multiple sclerosis plaque (×150). (t) Normal white matter of a control patient without neurological disease or brain lesions; no MDA2 immunoreactivity (×900). (u, v) MDA2 immunoreactivity in oligodendrocytes and myelin in active multiple sclerosis lesions; the nature of the cells as oligodendrocytes is shown by double staining in v, using TPPP-p25 as a marker (×900). (w) Granular cytoplasmic MDA2 immunoreactivity (blue) in the cytoplasm of a glial fibrillary acidic protein (GFAP)-positive astrocyte (red) in an active multiple sclerosis lesion (×800). LFB = Luxol fast blue.