Abstract
Degeneration of the dopaminergic nigrostriatal system and of noradrenergic neurons in the locus coeruleus are important pathological features of Parkinson’s disease. There is an urgent need to develop therapies that slow down the progression of neurodegeneration in Parkinson’s disease. In the present study, we tested whether the highly specific metabotropic glutamate receptor 5 antagonist, 3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] pyridine, reduces dopaminergic and noradrenergic neuronal loss in monkeys rendered parkinsonian by chronic treatment with low doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Weekly intramuscular 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine injections (0.2–0.5 mg/kg body weight), in combination with daily administration of 3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] pyridine or vehicle, were performed until the development of parkinsonian motor symptoms in either of the two experimental groups (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] pyridine versus 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/vehicle). After 21 weeks of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment, all 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/vehicle-treated animals displayed parkinsonian symptoms, whereas none of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] pyridine-treated monkeys were significantly affected. These behavioural observations were consistent with in vivo positron emission tomography dopamine transporter imaging data, and with post-mortem stereological counts of midbrain dopaminergic neurons, as well as striatal intensity measurements of dopamine transporter and tyrosine hydroxylase immunoreactivity, which were all significantly higher in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] pyridine-treated animals than in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/vehicle-treated monkeys. The 3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] pyridine treatment also had a significant effect on the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced loss of norepinephrine neurons in the locus coeruleus and adjoining A5 and A7 noradrenaline cell groups. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/vehicle-treated animals, almost 40% loss of tyrosine hydroxylase-positive norepinephrine neurons was found in locus coeruleus/A5/A7 noradrenaline cell groups, whereas the extent of neuronal loss was lower than 15% of control values in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] pyridine-treated monkeys. Our data demonstrate that chronic treatment with the metabotropic glutamate receptor 5 antagonist, 3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] pyridine, significantly reduces 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity towards dopaminergic and noradrenergic cell groups in non-human primates. This suggests that the use of metabotropic glutamate receptor 5 antagonists may be a useful strategy to reduce degeneration of catecholaminergic neurons in Parkinson’s disease.
Keywords: substantia nigra, locus coeruleus, striatum, neuroprotection, noradrenaline
Introduction
Parkinson’s disease is a progressive neurodegenerative disorder clinically characterized by bradykinesia, tremor, rigidity and postural instability. In addition, non-motor manifestations are increasingly recognized as being part of the wider clinical syndrome of Parkinson’s disease (Aarsland et al., 1999, 2004; Braak et al., 2003; Zesiewicz et al., 2003; Grimbergen et al., 2004; Langston, 2006). Although there is compelling evidence that the parkinsonian motor symptoms are largely due to the progressive degeneration of the nigrostriatal dopaminergic system, the pathophysiological substrate of non-motor deficits is unknown. It is possible that some of these deficits result from degeneration of noradrenergic neurons in the locus coeruleus and adjoining areas (Braak et al., 2003; Remy et al., 2005; Fornai et al., 2007; Benarroch, 2009; Frisina et al., 2009; Barone, 2010), a pathological feature described in post-mortem brain studies of patients with Parkinson’s disease (Halliday et al., 1990; Chan-Palay 1991; German et al., 1992; Patt and Gerhard 1993; Del Tredici et al., 2002; Braak et al., 2003; Zarow et al., 2003; Fornai et al., 2007). In addition, rodent studies have suggested that degeneration of norepinephrine neurons in the locus coeruleus may precede and could contribute to the degeneration of dopaminergic cells in the ventral midbrain (Marien et al., 1993; Fornai et al., 1995, 1997, 2007; Rommelfanger et al., 2007a, b).
The development of therapeutic approaches that could slow down the death of midbrain dopaminergic neurons has been of great interest during the past decade (Clarke, 2004; Schapira and Olanow, 2004; Biglan and Ravina, 2007; Kieburtz and Ravina, 2007; Schapira, 2008; Olanow, 2009; Pavese et al., 2009; Yacoubian and Standaert, 2009). Although the mechanisms that underlie nigral dopaminergic cell loss in Parkinson’s disease remain unknown (and may differ between patients), there is preclinical evidence that blockade of ionotropic glutamate receptors [i.e. 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4l)-propanoic acid and N-methyl-d-aspartic acid] protects midbrain dopaminergic neurons from toxin-induced degeneration in rodent and non-human primate models of Parkinson’s disease (Turski et al., 1991; Brouillet and Beal, 1993; Srivastava et al., 1993; Loschmann et al., 1994; Ossowska, 1994; Kanthasamy et al., 1997; Lange et al., 1997; Sonsalla et al., 1998; Konitsiotis et al., 2000; Horowitz and Greenamyre, 2010). However, presumably due to the fact that these fast acting glutamate receptors are essential for normal brain operations, chronic administration of ionotropic glutamate receptor antagonists elicits unwanted side effects in humans, thereby limiting their usefulness as therapeutics (Marino et al., 2003; Muir, 2006; Johnson et al., 2009).
Because of their modulatory effects, localization specificity and potential for drug targeting at allosteric modulatory sites, the G protein-coupled metabotropic glutamate receptors have generated significant interest as new therapeutic targets for brain diseases, including Parkinson’s disease (Conn et al., 2005; Ossowska et al., 2007; Gasparini et al., 2008; Nicoletti et al., 2010; Niswender and Conn, 2010). There is evidence that antagonists of metabotropic glutamate receptor 5 (mGluR5), a member of the group I metabotropic glutamate receptors, have significant antidyskinetic effects in rodent and non-human primate models of Parkinson’s disease (Levandis et al., 2008; Rylander et al., 2009, 2010; Morin et al., 2010; Johnston et al., 2010). Furthermore, mGluR5 receptor antagonists have antiparkinsonian effects in 6-hydroxydopamine-treated rats (Breysse et al., 2002), and reduce the toxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) against midbrain dopaminergic neurons in mice (Battaglia et al., 2004; Aguirre et al., 2005; Armentero et al., 2006; Vernon et al., 2007).
In light of these promising findings, the present study addressed the potential neuroprotective effects of the highly specific mGluR5 antagonist, 3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] (MTEP), against the loss of midbrain dopaminergic neurons in a chronic MPTP-treated monkey model of Parkinson’s disease. Furthermore, taking into consideration that the chronic MPTP monkey model used in this study also results in damage to locus coeruleus noradrenergic cells (Masilamoni et al., 2010b), combined with the fact that locus coeruleus neurons display functional mGluR5 expression (Page et al., 2005; Rasmussen et al., 2005; Noriega et al., 2007), and that lesion of noradrenergic systems may underlie some of the non-motor symptoms of Parkinson’s disease (Remy et al., 2005; Fornai et al., 2007; Benarroch, 2009; Frisina et al., 2009), another objective of this study was to assess whether MTEP may also protect brainstem noradrenergic neurons in the locus coeruleus and adjoining cell groups against the toxic effects of chronic MPTP injections.
Some of the data reported here have been presented in abstract forms (Masilamoni et al., 2009, 2010a, b).
Materials and methods
Animals
Ten adult female rhesus monkeys (Macaca mulatta, 4.5–8.5 kg) from the Yerkes National Primate Research Center colony were used in this study, in accordance with guidelines from the National Institutes of Health. All procedures were approved by Emory’s Animal Care and Use Committee. The animals were housed in a temperature-controlled room and exposed to a 12-h light/dark cycle. They were fed twice daily with monkey chow supplemented with fruits or vegetables. The animals had free access to water.
Experimental design
The 10 rhesus monkeys used in this study were divided into three groups. Group 1 consisted of three animals that were treated with MPTP and vehicle, Group 2 consisted of four animals treated with MPTP and MTEP, and Group 3 was comprised of three untreated monkeys that were used for tyrosine hydroxylase and dopamine transporter immunocytochemistry, and for stereological cell counting.
The temporal sequence of the experimental procedures is shown in Fig. 1. The seven monkeys in Groups 1 and 2 were first trained to sit in a primate chair, and transported to a behavioural testing room once weekly to habituate them to the testing environment. After collection of baseline behavioural data using methods described below, these monkeys received injections of either vehicle (Group 1) or MTEP (10 mg/kg, intramuscular, Group 2) for 5 weeks prior to the beginning of MPTP treatments, to assess the potential effects of mGluR5 blockade on motor behaviour and on 2β-carbomethoxy-3β-(4-chlorophenyl)-8-(2-[18F]-fluoroethyl)-nortropane (18F-FECNT) positron emission tomography (PET) imaging in naïve monkeys. The MTEP dosage was chosen based on pilot data from our laboratory (Bogenpohl et al., 2006) and others showing that MTEP monotherapy is well tolerated and achieves maximum antiparkinsonian and antidyskinetic effects at 10 mg/kg (Phillips et al., 2006; Morin et al., 2010).
Figure 1.
Study design.
A series of weekly intramuscular MPTP injections (0.2–0.5 mg/kg body weight; Sigma-Aldrich) was then started (i.e. Week 5 in Fig. 1). This regimen was continued until significant parkinsonian motor symptoms appeared in all animals of either treatment groups. To avoid any possible unknown effects MTEP may have on MPTP pharmacokinetics and metabolism, the MTEP daily administration was performed at least 12 h prior to any weekly injections of MPTP. After 18–21 weeks of MPTP treatment, (i.e. Weeks 23–26 in Fig. 1) all Group 1 animals displayed moderate to severe parkinsonian symptoms according to the rating scale described below. In contrast, none of the MTEP-treated monkeys in Group 2 showed any significant change in their motor behaviour relative to baseline measurements (Fig. 2). At this point, the MPTP administration (cumulative dose of 7.9 mg MPTP/kg body weight) was terminated in all monkeys, followed by 2 weeks of MTEP washout before a final assessment of Parkinson’s disease symptoms, to ensure that the differences in parkinsonian motor scores resulted from a variable degree of nigrostriatal degeneration instead of the symptomatic antiparkinsonian effects of MTEP between the two groups. The MTEP administration was then initiated again for one more week to ensure that all animals had been recently exposed to the drug treatment at the time of sacrifice (Fig. 1).
Figure 2.
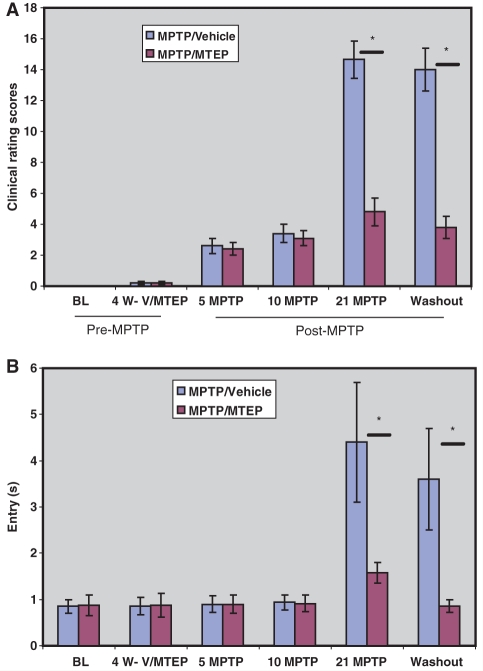
Histograms illustrating the progressive increase of (A) Parkinsonism rating scores and (B) food picking ‘entry’ time (i.e. the time between entering the apparatus and retrieval of a food object) in MPTP-treated monkeys (mean ± SD). Four weeks of vehicle (V) or MTEP (10 mg/kg) treatment (4 W-V/MTEP) did not alter behaviour. Significant motor impairments were observed in MPTP/vehicle-treated monkeys after 21 weekly MPTP injections, in the vehicle-treated group (‘21 MPTP’) and were maintained throughout the rest of the study period. *P < 0.001 for differences between the vehicle and MTEP-treated animals. No significant difference was found between control and MPTP/MTEP- treated monkeys. BL = baseline.
Positron emission tomography neuroimaging
In vivo PET imaging with dopamine transporter ligands is the most sensitive approach to follow longitudinally the state of degeneration of the nigrostriatal dopaminergic system during the course of neuroprotective trials in Parkinson’s disease (see review in Pavese and Brooks, 2009; Pavese et al., 2009). We have recently shown that the PET tracer 18F-FECNT, is a highly reliable ligand to estimate levels of striatal and nigral dopamine transporter in the MPTP-treated monkey model of Parkinson’s disease (Masilamoni et al., 2010a). In order to further characterize the use of this ligand as a reliable indicator of the degree of striatal and nigral dopaminergic denervation in MPTP-treated monkeys (Masilamoni et al., 2010b), and to follow dynamic changes of the nigrostriatal dopaminergic system during the course of MPTP intoxication (±MTEP), each monkey underwent five 18F-FECNT PET scans in these experiments (Fig. 1). First, we assessed possible changes in 18F-FECNT binding induced by MTEP prior to the beginning of MPTP administration with two PET scans (PET I and PET II) that were performed 10 weeks apart before MPTP treatment. PET I was performed prior to the beginning of any behavioural training or drug treatment. Then, baseline behavioural data were collected for five weeks prior to the beginning of MTEP or vehicle administration (i.e. Week 0 in Fig. 1). During this period, Group 1 animals received daily injections of vehicle, while Group 2 monkeys were injected daily with MTEP for 4 weeks prior to the second PET scan (PET II) and the beginning of the MPTP treatment. The third and fourth PET scans (PET III and PET IV) were performed 5 and 10 weeks after the start of the MPTP treatment regimen (i.e. Weeks 9 and 14 in Fig. 1) to monitor changes in striatal dopamine transporter binding. The fifth PET scan (PET V) was performed 21 weeks after the start of the MPTP treatment protocol (i.e. Week 26 in Fig. 1), after the development of parkinsonian symptoms in all MPTP/vehicle-treated monkeys.
PET images were acquired with a Siemens Focus 220 micro-PET scanner. The monkeys were fasted for 12 h prior to the PET scans. The animals were intubated under anaesthesia with Telazol (3 mg/kg intramuscular), and then maintained throughout the imaging session on a mixture of 1% isoflurane and 5% oxygen gas. The monkeys were positioned in the scanner and connected to monitoring equipment to measure respiratory parameters, blood pressure, pulse rate and rectal temperature. A 15-min transmission scan was obtained for attenuation correction, then a slow bolus of ∼5.0 mCi of 18F-FECNT (specific activity 1.5 Ci/μmol) was injected over 5–6 min at a rate of 1.0 ml/min. The collection of scans began at the same time as the start of the radiotracer injection. The initial acquisition was a 28-frame dynamic sequence, starting with 30-s scans and ending with 20-min scans (total duration, 110 min). All images were reconstructed using the manufacturer-supplied software with measured attenuation correction, zoom factor 8, and Shepp–Logan reconstruction with a filter cut-off at 1 cycle/cm. The axial slice thickness was 3.375 mm. All images were decay-corrected to the time of injection. For the generation of time-activity curves for each monkey, the five different PET images taken in different experimental conditions (PET I–V) were superimposed using IDL software (ITT Visual Information Solutions), and averaged to draw regions of interest so that the volume would be equal and comparable. Regions of interest were manually drawn on the average image, delimiting the following striatal regions: putamen/associative, putamen/motor, putamen/limbic, caudate nucleus/associative, nucleus accumbens/limbic and the substantia nigra. The regions of interest were then superimposed onto the individual images to obtain time-activity curves. Because of the minimal expression of dopamine transporter in the cerebellum, we used it as the reference region, and expressed the FECNT uptake value as region of interest/cerebellum ratio, as described in our previous studies (Votaw et al., 2002; Masilamoni et al., 2010a). One-way ANOVA was used to determine significance of differences between control, MPTP/vehicle and MPTP/MTEP groups.
Behavioural observations
Changes in the animal’s behavioural state were documented through observations in an observation cage of which one of the side walls was made of Plexiglas for easy visibility of the monkey. After having been brought to this cage, the animals were given a 30-min habituation period before being videotaped for 15 min/session. Their behaviour was later monitored and evaluated from videos viewed by two observers, one of them blinded to the treatment regimen, using a modified Parkinson’s disease rating scale. The differences in the rating scores between the two observers were <6%. The mean values obtained by the two experimenters were used for statistical analysis. The scale used in this study evaluated nine criteria (gross motor activity, balance, posture, arm bradykinesia, arm hypokinesia, leg bradykinesia, leg hypokinesia, arm tremor and leg tremor). Each criterion received a score between 0 and 3 (0 = normal, 1 = mild, 2 = moderate, 3 = severe), for a maximum of 27 points. The total number of points was used as the clinical score to compare the severity of parkinsonian motor symptoms across animals in the different experimental groups.
In addition, the monkeys were trained to perform a food retrieval task to assess their fine motor skills. During this test, animals were required to retrieve a raisin from a device that uses infrared beams to automatically measure the time required for the monkey to move its hand from the door to the raisin (entry) and from the raisin back to the door (exit). A minimum of 10 trials per hand was performed in each session. The average entry and exit times to perform this test were calculated from data collected in both hands because there was no significant difference in this test performance between the right and left hand.
Because of the rapid clearance of MTEP (Anderson et al., 2003; Busse et al., 2004; Johnston et al., 2010), the behavioural tests were performed between 20 and 24 h after the daily MTEP injections to avoid any confound in the test performance induced by possible acute antiparkinsonian effects of MTEP.
Termination of the experiments
At the end of the experiments, the monkeys were deeply anaesthetized with an overdose of pentobarbital (100 mg/kg, intravenous), and perfused transcardially with cold oxygenated Ringer’s solution, followed by 2 l of fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in phosphate buffer (0.1 M, pH 7.4). After perfusion, the brains were removed from the skull and cut into 10 mm-thick blocks in the frontal plane. The blocks were further cut into 50 µm-thick sections with a vibratome and used for post-mortem immunostaining and cell counting procedures.
Immunostaining
To validate the extent of MTEP effects upon MPTP-induced degeneration of the dopaminergic nigrostriatal system, striatal and midbrain sections of untreated, MPTP/vehicle- and MPTP/MTEP-treated monkeys were immunostained with antibodies against dopamine transporter (monoclonal rat anti-dopamine transporter IgGs) or tyrosine hydroxylase (monoclonal mouse anti-tyrosine hydroxylase IgGs) at a concentration of 1:1000 (Millipore; catalogue numbers MAB 369 and 318, respectively). In addition, we used highly specific mouse monoclonal calbindin D28K antibodies at a concentration of 1:4000 (Sigma, catalogue number C9848) to label calbindin-positive cells in the dorsal tier of the substantia nigra pars compacta and the ventral tegmental area, which allowed us to differentiate these neurons from the calbindin-negative ventral tier substantia nigra pars compacta neurons (Masilamoni et al., 2010a). The immunoperoxidase avidin biotin complex method with diaminobenzidine as chromogen was used to localize the different markers according to procedures described in previous studies (Hsu et al., 1981; Smith and Bolam, 1991). Sections were then rinsed in phosphate-buffered saline (0.01 M, pH 7.4), mounted onto gelatin-coated slides, dehydrated and cover-slipped with Permount. Dopamine transporter and tyrosine hydroxylase immunoreactivity in the striatum was quantified using the MCID program (Imaging Research Inc.). Each slide was illuminated using an even-field illumination table, and macroscopic images were acquired with a Cool SNAP cf photometrics camera. The regions of interest were outlined on a computer screen, and the average pixel density measured and expressed as relative optical density. Mean values were calculated from five to seven sections per hemisphere. The SigmaStat software (Systat) was used to assess the significance of differences in labelling intensity measured from the various striatal regions of interest in the MPTP/vehicle- and MPTP/MTEP-treated animals.
Stereological estimation of tyrosine hydroxylase-positive midbrain, locus coeruleus, A5 and A7 noradrenaline neurons
The unbiased stereological estimation of the number of dopamine neurons in the ventral substantia nigra pars compacta, dorsal substantia nigra pars compacta, ventral tegmental area, and norepinephrine neurons in locus coeruleus, A5, A7 noradrenaline cells was achieved using the optical fractionator principle (StereoInvestigator, MicroBrightField, Inc.), a stereological approach that combines the optical dissector with a fractionator sampling scheme. This sampling technique is not affected by tissue volume changes and does not require reference volume determinations. The random systematic sampling of counting areas was done using the Leica DMR microscope. To count midbrain tyrosine hydroxylase-positive neurons, we first took low power micrographs (×1.25) of tyrosine hydroxylase- and calbindin-immunostained ventral midbrain sections, and manually delineated the borders of the ventral substantia nigra pars compacta, dorsal substantia nigra pars compacta, and ventral tegmental area, based on the presence or absence of calbindin-positive neurons (Masilamoni et al., 2010a). Then, the borders of the different ventral midbrain regions were manually delineated on tyrosine hydroxylase-immunolabelled slides adjacent to those immunostained for calbindin. The noradrenergic cell groups were delineated based on the expression of tyrosine hydroxylase-immunoreactive neurons using the following anatomical landmarks: (i) locus coeruleus: tyrosine hydroxylase-positive neurons located above the dorsal limit of the medial longitudinal fasciculus medial to the superior cerebellar peduncle; (ii) A7 noradrenaline cells: tyrosine hydroxylase-positive neurons located between the dorsal border of medial longitudinal fasciculus and the medial olivary nucleus; and (iii) A5 noradrenaline cells: tyrosine hydroxylase-positive neurons surrounding the medial olivary nucleus (Fig. 8A). Counts of tyrosine hydroxylase-positive cells were generated using a ×100 oil-immersion objective. To perform unbiased stereology, counting frames (65 × 65 µm) were randomly placed by the stereology software within the chosen region of interest. The software also controlled the position of the x–y stage of the microscope, so that the entire brain region could be scanned by successively meandering between counting frames. On average, 12 sections were analysed and ∼300 cells were counted. The software calculated the estimated total number of cells in each region of interest per hemisphere. The cell counts were performed by two investigators, one of them blinded to the drug treatment. The differences in the total number of neurons counted by these investigators were <5%. The mean of values obtained by each experimenter was used for statistical analysis.
Figure 8.
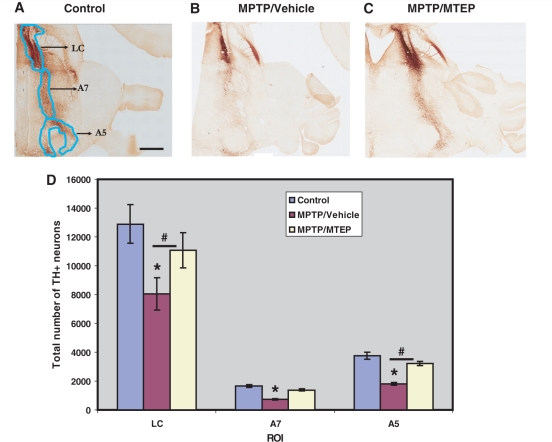
Tyrosine hydroxylase labelling in noradrenergic cells groups. (A) Control, (B) MPTP/vehicle, (C) MPTP/MTEP-treated monkeys. (D) Stereological estimate of the total number of tyrosine hydroxylase-positive (TH+) neurons (means ± SD) in locus coeruleus, A5 and A7 regions of control, MPTP/vehicle and MPTP/MTEP-treated monkeys, representing noradrenergic neurons. *P < 0.05 for differences between control, MPTP/vehicle- and MPTP/MTEP-treated animals. #P < 0.05 for differences between the vehicle and MTEP-treated animals. LC = locus coeruleus; A5 and A7 = catecholaminergic areas A5 and A7; ROI = region of interest. Scale bar = 5 mm (A–C).
Results
Comparison of changes in striatal 18F-2β-carbomethoxy-3β-(4-chlorophenyl)-8-(2-[18F]-fluoroethyl)-nortropane positron emission tomography imaging data with changes in behaviour
PET I served to determine the baseline 18F-FECNT uptake values in the striatum and the substantia nigra of each animal that received subsequent MPTP injections, while PET II served to assess potential effects of MTEP treatment upon 18F-FECNT binding. This scan was performed 20–24 h after the last MTEP (or vehicle) administration, to avoid possible acute MTEP effects upon FECNT binding. The uptake values measured in PET II were not significantly different from those obtained under baseline conditions (PET I) (Figs 3D and E, 4D–F and 5D), indicating that MTEP alone does not significantly affect 18F-FECNT uptake measurements at the striatal and midbrain levels. Four weeks of MTEP treatment also did not alter scores on the parkinsonian rating scale, or performance in the food picking task in these monkeys (Fig. 2A and B).
Figure 3.
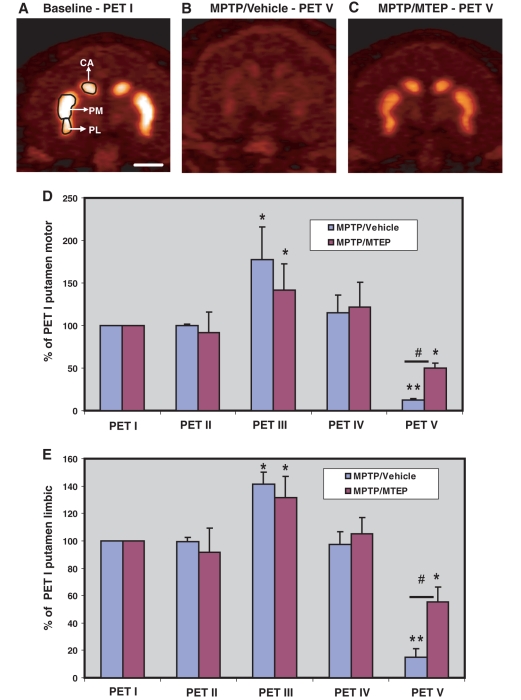
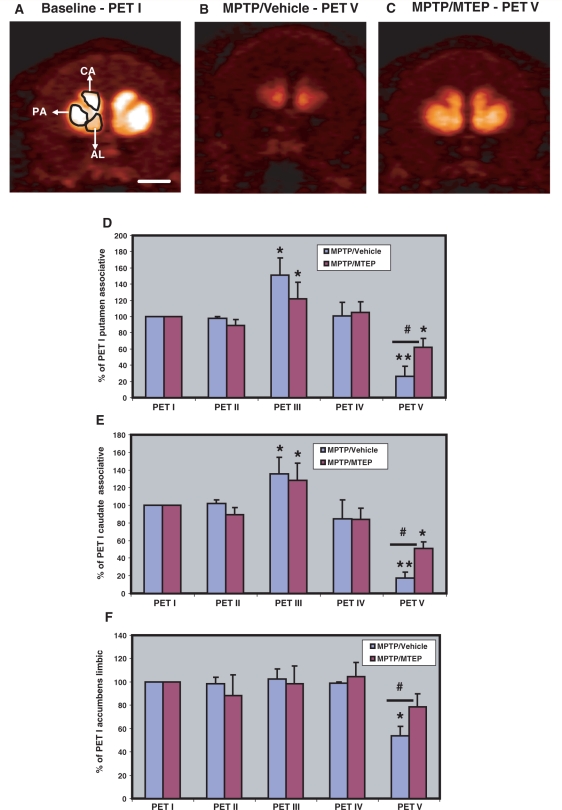
Representative post-commissural coronal in vivo 18F-FECNT PET images. (A) Baseline, (B) MPTP/vehicle, (C) MPTP/MTEP-treated monkeys. (D, E) Bar graphs showing the percentages of striatocerebellar 18F-FECNT uptake ratios (means ± SD) for the motor putamen (D) and the limbic putamen (E), obtained from the five different PET scans collected throughout the study. **P < 0.001; *P < 0.05 for differences between control, MPTP/vehicle- and MPTP/MTEP-treated animals. #P < 0.05 for differences between vehicle- and MTEP-treated animals. CA = associative caudate nucleus; PM = motor putamen; PL = limbic putamen. Scale bar = 10 mm (A–C).
Figure 4.
Representative pre-commissural coronal planes of in-vivo 18F-FECNT PET images. (A) Baseline, (B) MPTP/vehicle, (C) MPTP/MTEP-treated monkeys. (D–F) Bar graphs showing the percentages of striatocerebellar 18F-FECNT uptake ratios (means ± SD) for the pre-commissural putamen (D), caudate nucleus (E) and the nucleus accumbens (F) obtained from the five PET scans throughout the study. **P < 0.001; *P < 0.05 for differences from control, MPTP/vehicle-, MPTP/MTEP-treated animals. #P < 0.05 for differences between the vehicle and MTEP-treated animals. No significant difference was found in the nucleus accumbens between control and MPTP/MTEP-treated monkeys. AL = limbic nucleus accumbens; CA = associative caudate nucleus; PA = associative putamen. Scale bar = 10 mm (A–C).
Figure 5.
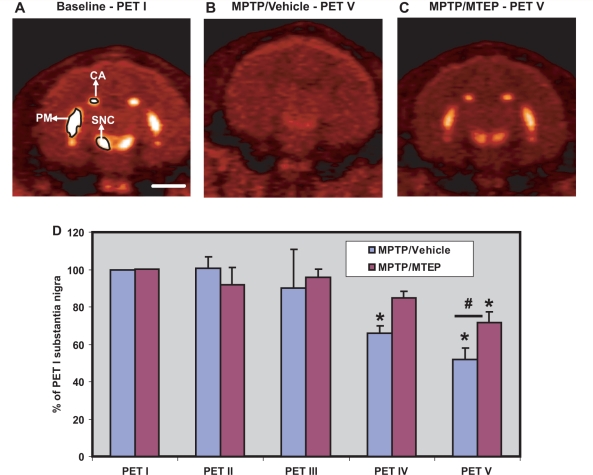
Representative midbrain coronal in-vivo 18F-FECNT PET images. (A) Baseline, (B) MPTP/vehicle, (C) MPTP/MTEP-treated monkeys. (D) Bar graph showing the percentage of nigrocerebellar 18F-FECNT uptake ratios (means ± SD) for the substantia nigra pars compacta obtained from the five PET scans throughout the study. *P < 0.05 for differences from control, MPTP/vehicle, MPTP/MTEP-treated animals. #P < 0.05 for differences between the vehicle and MTEP-treated animals. CA = associative caudate nucleus; PM = motor putamen; SNC = substantia nigra pars compacta. Scale bar = 10 mm (A–C).
However, the subsequent PET scan (PET III), performed 5 weeks after the beginning of weekly MPTP treatment, revealed a significant increase (P < 0.001) in 18F-FECNT uptake in all striatal regions of interest, except in the nucleus accumbens (nucleus accumbens/limbic) in the MPTP/vehicle- and MPTP/MTEP-treated animals (Figs 3D and E and 4D–F). This increase in PET measurement was not associated with changes in the parkinsonism rating score and performance in the food picking task (Fig. 2A and B). The transient increase in FECNT uptake was no longer apparent in the next PET scan, performed 5 weeks later (PET IV; Figs 3D and E and 4D and E).
After 18–21 weeks of MPTP treatment, all MPTP/vehicle-treated monkeys displayed moderate to severe parkinsonian symptoms, while monkeys treated with MPTP/MTEP did not display significant changes in their parkinsonism rating scores and performance in the food picking task (Fig. 2A and B). The different behavioural response to MPTP treatment was correlated with a significantly lower (P < 0.001) level of 18F-FECNT uptake in all striatal regions of interest in MPTP/vehicle- than in MPTP/MTEP-treated monkeys (PET V; Figs 3 and 4). It is worth noting that the striatal 18F-FECNT uptake values were below the baseline values in both MPTP/vehicle- and MPTP/MTEP-treated animals at this time point, suggesting that the daily MTEP treatment did not completely prevent a decrease in striatal dopamine transporter binding (Figs 3 and 4).
Effects of 3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] pyridine treatment on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced changes in midbrain 18F-2β-carbomethoxy-3β-(4-chlorophenyl)-8-(2-[18F]-fluoroethyl)-nortropane binding
The pattern of changes of 18F-FECNT uptake in the substantia nigra pars compacta/ventral tegmental area complex over time was slightly different from what was found in the striatum. Specifically, there was no transient increase of uptake measured in PET III in the substantia nigra pars compacta/ventral tegmental area region (Fig. 5). In addition, MPTP/vehicle-treated monkeys displayed a significant decrease in FECNT uptake (P < 0.05) in the substantia nigra pars compacta/ventral tegmental area, compared to the baseline in PET IV. Such a change was not observed in MPTP/MTEP-treated monkeys (Fig. 5D). In the final PET scan (PET V), both groups of animals displayed lower substantia nigra pars compacta/ventral tegmental area 18F-FECNT uptake than at baseline, with a greater decrease in the MPTP/vehicle-treated animals (Fig. 5). However, the differences in uptake values between the two groups were not as pronounced in the substantia nigra pars compacta as they were in the striatum (compare PET V values in Fig. 5D with Figs 3D and E and 4D–F).
3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] pyridine-induced protection of dopaminergic neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-mediated neurotoxicity
To determine whether the lack of parkinsonism in MPTP/MTEP-treated animals after 21 weeks of MPTP treatment was due to MTEP treatment-related preservation of the nigrostriatal dopaminergic denervation, or antiparkinsonian effects of MTEP (Bogenpohl et al., 2006), we compared the various behavioural measures taken after 21 weeks of MPTP treatment (i.e. Week 26 in Fig. 1) with those obtained following a 2 weeks washout of MTEP (i.e. Week 30 in Fig. 1). These data revealed no significant difference in behavioural scores between the two time points for either experimental group (Fig. 2), suggesting that the MTEP effects were not due to its symptomatic antiparkinsonian properties.
Post-mortem assessment of nigrostriatal dopaminergic denervation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/vehicle and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] pyridine-treated animals
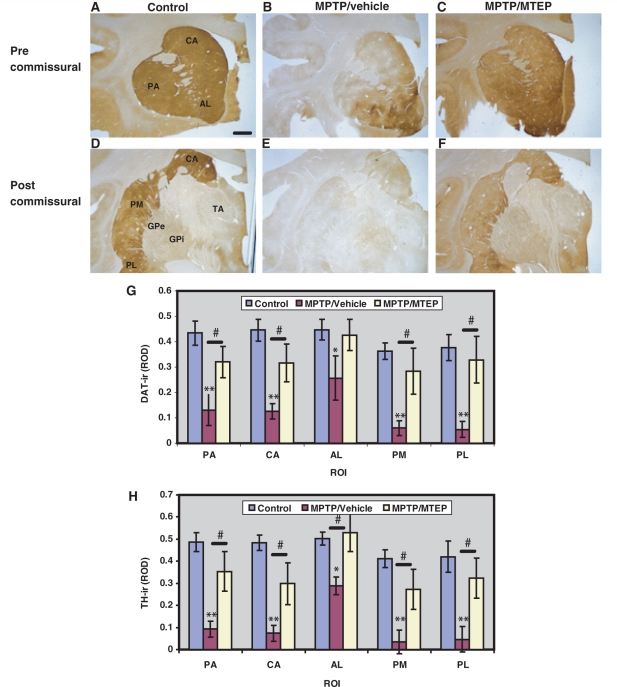
Brain sections from perfusion-fixed monkeys in each group were processed for light microscopic localization of tyrosine hydroxylase and dopamine transporter immunoreactivity. The data presented in Fig. 6A–F show different pre- and post-commissural striatal territories that were immunostained with a dopamine transporter antibody from control (Fig. 6A and D), MPTP/vehicle- (Fig. 6B and E) and MPTP/MTEP-treated (Fig. 6C and F) animals. MPTP/vehicle-treated animals displayed a significantly more severe decrease in striatal dopamine transporter immunoreactivity in all regions of interest compared with the MPTP/MTEP-treated monkeys (Fig. 6A–G). The degree of dopamine transporter depletion in the MPTP/vehicle-treated monkeys was most severe in the post-commissural putamen regions of interest (putamen/motor, putamen/limbic) (Fig. 6G). Similar findings were found in striatal tissue immunostained for tyrosine hydroxylase (Fig. 6H).
Figure 6.
Representative coronal sections of pre-commissural (A–C) and post-commissural (D–F) striatum showing dopamine transporter (DAT) immunoreactivity in Controls, MPTP/vehicle and MPTP/MTEP-treated monkeys. (G–H) Densitometry analysis of control, MPTP/vehicle and MPTP/MTEP-treated monkeys. *P < 0.001 and **P < 0.05 for differences between control and MPTP/vehicle-treated monkeys. #P < 0.05 for differences between the vehicle and MTEP-treated animals. No significant difference was found between control and MPTP/MTEP-treated monkeys. AL = limbic nucleus accumbens; CA = associative caudate nucleus; GPe = external pallidal segment; GPi = internal pallidal segment; PA = associative putamen; PL = limbic putamen; PM = motor putamen; ROD = relative optical density; ROI = region of interest; TA = thalamus; TH-ir = thyrosine hydroxylase immunoreactivity. Scale bar = 5 mm (A–F).
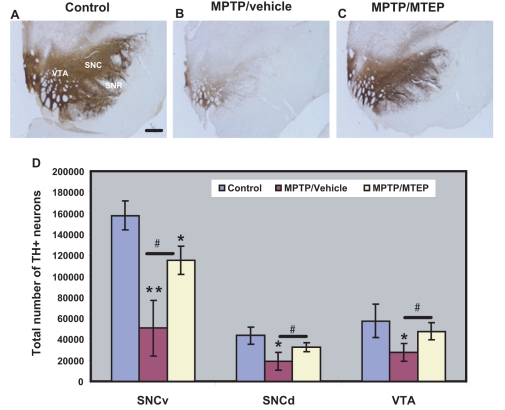
The degree of tyrosine hydroxylase-positive cell loss in the ventral midbrain, assessed with unbiased stereological cell counts, was different across the three groups of dopaminergic neurons examined (ventral substantia nigra pars compacta, dorsal substantia nigra pars compacta, ventral tegmental area). In the ventral substantia nigra pars compacta, both MPTP/vehicle- and MPTP/MTEP-treated monkeys displayed a significant loss of tyrosine hydroxylase-immunoreactive neurons compared with controls (P < 0.001). In the dorsal substantia nigra pars compacta and ventral tegmental area, a significant loss of tyrosine hydroxylase-immunoreactive neurons was only seen in the MPTP/vehicle-treated animals (P < 0.05). The decrease in the number of tyrosine hydroxylase-immunoreactive neurons was significantly more pronounced (P < 0.001) in the MPTP/vehicle-treated monkeys than in MPTP/MTEP-treated animals in all midbrain dopaminergic cell groups (ventral substantia nigra pars compacta: 68 ± 17% loss in vehicle-treated animals versus 31 ± 8% loss in MTEP-treated animals; dorsal substantia nigra pars compacta: 57 ± 21% versus 25 ± 10 %; ventral tegmental area: 48 ± 14% versus 11 ± 13%) (Fig. 7A–D).
Figure 7.
Photomicrographs showing tyrosine hydroxylase labelling in the mesencephalon. (A) Control, (B) MPTP/vehicle, (C) MPTP/MTEP-treated monkeys. (D) Stereological estimate of the total number of tyrosine hydroxylase-positive (TH+) neurons (means ± SD) in the ventral substantia nigra pars compacta, dorsal substantia nigra pars compacta and ventral tegmental area regions of control, MPTP/vehicle and MPTP/MTEP-treated monkeys. *P < 0.001; **P < 0.05 for differences between control, MPTP/vehicle and MPTP/MTEP-treated animals by using one-way ANOVA with Tukey’s post hoc test. There was no significant difference found in the dorsal substantia nigra pars compacta and ventral tegmental area between control and MPTP/MTEP-treated monkeys. SNCd = dorsal substantia nigra compacta; SNCv = ventral substantia nigra compacta; SNR = substantia nigra pars reticulata; VTA = ventral tegmental area. Scale bar = 5 mm (A–C).
3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] pyridine-mediated protection of noradrenergic neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity
Taking advantage of the chronic MPTP regimen used in the present study that induces significant loss of noradrenergic neurons in the locus coeruleus and related A5 and A7 noradrenaline cell groups (Masilamoni et al., 2010 b), we determined the effect of MTEP treatment towards noradrenergic cell loss in MPTP-treated monkeys. As shown in Fig. 8, MPTP treatment resulted in a more significant reduction of the number of tyrosine hydroxylase-positive neurons in the locus coeruleus, A5 and A7 noradrenaline cells in vehicle-than in MTEP-treated animals (locus coeruleus: 38.7 ± 8.8% loss in vehicle-treated animals versus 15.2 ± 9.4% loss in MTEP-treated animals; A5 noradrenaline cells: 48.3 ± 7.2% versus 13.8 ± 3.7%; A7 noradrenaline cells: 45.1 ± 7.8 versus 16.4 ± 4.7%). Although the percentage of cell loss in MPTP/vehicle-treated animals was most severe in the locus coeruleus, it reached significance in all neuronal groups (P < 0.001). The total number of labelled neurons in the MPTP/MTEP-treated animals was not significantly different from the controls (Fig. 8; P > 0.05).
Discussion
The present study shows that the mGluR5 antagonist, MTEP, has significant protective effects against MPTP-induced degeneration of midbrain dopaminergic neurons and noradrenergic locus coeruleus/A5/A7 neurons in non-human primates. Together, these findings suggest that mGluR5 receptor antagonists may be useful agents to delay the demise of dopaminergic and noradrenergic systems in Parkinson’s disease. Furthermore, our data demonstrate that the dopamine transporter ligand, 18F-FECNT is a sensitive and highly reliable biomarker to assess the state of the striatal dopamine innervation and the extent of midbrain dopaminergic cell loss during the course of nigrostriatal dopaminergic denervation in parkinsonism (Masilamoni et al., 2010a).
Potential mechanisms by which metabotropic glutamate receptor 5 antagonists may protect midbrain dopaminergic neurons from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in monkeys
The present data provide neuropathological and behavioural evidence that prolonged systemic administration of the mGluR5 specific antagonist, MTEP, protects dopaminergic neurons in the substantia nigra pars compacta/ventral tegmental area against MPTP-induced degeneration in rhesus monkeys. These findings are consistent with previous data showing that mice treated with the mGluR5 receptor antagonist MPEP, or mice that lack the mGluR5 receptor gene, are significantly protected against MPTP-induced nigrostriatal dopamine denervation (Battaglia et al., 2004). The exact mechanisms underlying these beneficial effects remain unknown, although various possibilities can be suggested. Thus, in light of the literature suggesting that calcium mishandling, mitochondrial respiration impairment and excitotoxicity contribute to the loss of midbrain dopaminergic cells in Parkinson’s disease (Sherer et al., 2002; Maesawa et al., 2004; Wallace et al., 2007; Caudle and Zhang 2009; Chan et al., 2009; Winklhofer and Haass 2009; Cannon and Greenamyre, 2010; Van Laar and Berman 2010), the blockade of mGluR5 on the surface of midbrain dopaminergic neurons (Hubert et al., 2001; Hubert and Smith, 2004) may provide its protective effects upon substantia nigra pars compacta neurons through a reduction of mGluR5-mediated intracellular calcium release from internal stores, one of the key mechanisms by which mGluR5 activation mediates its excitatory effects in various neuronal populations (Kim et al., 1994; Pin and Duvoisin, 1995; Conn and Pin, 1997; Nakanishi et al., 1998; Sala et al., 2005; Zhang et al., 2005). However, this hypothesis awaits confirmation of intracellular calcium increase in response to mGluR5 activation on nigral dopaminergic neurons. In addition to these postsynaptic effects, mGluR5 blockers may also reduce the activity of glutamatergic inputs to the substantia nigra pars compacta, specifically those from subthalamic nucleus neurons, which strongly respond to mGluR5 activation and display abnormal increased activity in parkinsonism (Rodriguez et al., 1998; Bezard et al., 1999; Awad et al., 2000; Breysse et al., 2003; Fazal et al., 2003; Shimo and Wichmann 2009; Piallat et al., 2011).
Another mechanism by which MTEP protection could be mediated is through the regulation of the potentiating effects of mGluR5 upon N-methyl-d-aspartic acid receptor function (Calabresi et al., 1998; Tu et al., 1999; Awad et al., 2000; Alagarsamy et al., 2002). Because N-methyl-d-aspartic acid receptors represent a main source of calcium entry in midbrain dopaminergic neurons, a reduced mGluR5-mediated potentiation of these receptors may have a significant impact on the level of intracellular calcium handled by dopaminergic neurons upon glutamatergic activation. In fact, N-methyl-d-aspartic acid receptor blockers have been considered as neuroprotective agents against toxin-induced degeneration of midbrain dopaminergic neurons in animal models of parkinsonism (Turski et al., 1991; Srivastava et al., 1993; Loschmann et al., 1994; Ossowska, 1994; Kanthasamy et al., 1997; Sonsalla et al., 1998), though their clinical use is limited due to unwanted side effects, as discussed above. Finally, knowing the significant glial expression of mGluR5 in the ventral midbrain (Hubert et al., 2001; Hubert and Smith, 2004), and its upregulation in some inflammatory processes (Byrnes et al., 2009; Loane et al., 2009; Drouin-Ouellet et al., 2011), the blockade of these receptors in astrocytes and microglia may modulate inflammatory processes that contribute to MPTP-induced toxicity towards midbrain dopaminergic neurons.
On the other hand, one must also consider the possibility that the MTEP-mediated neuroprotective effects could have been induced by interference of MTEP with the conversion mechanisms of MPTP into the toxin 1-methyl-4-phenylpyridinium+, thereby reducing exposure of dopaminergic and noradrenergic terminals in MTEP-treated animals to toxic levels of 1-methyl-4-phenylpyridinium+. However, such interfering mechanism is very unlikely based on recent mouse data showing that MPEP administration 30 min prior to MPTP (10 mg/kg) injections does not have any significant effect on the intrastriatal levels of 1-methyl-4-phenylpyridinium+ measured at various time points in vivo from striatal homogenates (Battaglia et al., 2004).
Biphasic regulation in striatal dopamine transporter binding during the course of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-mediated nigrostriatal dopaminergic degeneration
Our longitudinal monitoring of striatal dopamine transporter binding along the course of MPTP treatment revealed a transient increase in 18F-FECNT uptake after 5 weeks of MPTP treatment in both MPTP/saline- and MPTP/MTEP-treated animals. To our knowledge, this represents the first evidence of increased dopamine transporter binding in the striatum of animals intoxicated with MPTP. However, dopamine transporter upregulation has previously been reported in mice and baboons treated with acute low doses of organochlorine insecticides, or manganese, respectively (Kirby et al., 2001; Chen et al., 2006). The underlying substrate of this increased PET signal remains unclear, but it is possible that the affinity of the remaining dopamine transporter for FECNT was transiently increased, that the synthesis of dopamine transporter in the remaining dopaminergic terminals was increased, or that dopamine transporter-containing dopaminergic axons and terminals showed an excessive degree of sprouting early in the course of degeneration of the nigrostriatal system (Song and Haber, 2000; Kirby et al., 2001; Gillette and Bloomquist, 2003; Chen et al., 2006). It is noteworthy that binding of D2-like dopamine receptors also shows a biphasic regulation during the course of MPTP intoxication in monkeys (Bezard et al., 2001), although in contrast to dopamine transporter, D2-like receptor binding shows an initial decrease followed by an upregulation (Bezard et al., 2001). Together, these findings demonstrate the complex and dynamic regulatory processes of the nigrostriatal dopaminergic system during MPTP-induced toxicity. It remains to be established whether such changes represent pathological or compensatory mechanisms in response to MPTP administration.
Metabotropic glutamate receptor 5 antagonist effects on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced degeneration of noradrenergic neurons
In addition to its protective effects against midbrain dopaminergic cell loss, MTEP significantly reduced the extent of norepinephrine cell loss in the locus coeruleus and the adjoining A5 and A7 noradrenaline cell groups in all chronically MPTP-treated monkeys examined in our study. Although loss of locus coeruleus neurons is a well recognized pathological feature of human Parkinson’s disease (Chan-Palay, 1991; German et al., 1992; Patt and Gerhard, 1993; Del Tredici et al., 2002; Braak et al., 2003; Zarow et al., 2003), the development of such pathology in animal models of parkinsonism has been controversial (Burns et al., 1983; Langston et al., 1984; Mitchell et al., 1985; Forno et al., 1986, 1993; Miyoshi et al., 1988; Gibb et al., 1989; Herrero et al., 1993; Rose et al., 1993). In light of our findings and those reported in mice implanted with MPTP-releasing mini pumps (Fornai et al., 2005), it appears that the regimen of MPTP administration used in these studies may account for the differences in norepinephrine cell loss, with chronic administration regimes favouring the loss of locus coeruleus neurons. In MPTP/vehicle-treated monkeys, our findings revealed a 40% decrease in the number of tyrosine hydroxylase-positive neurons in the locus coeruleus/A5/A7 noradrenaline cell groups. However, only about 15% loss was found in animals that received MTEP treatment, suggesting that MTEP protected the norepinephrinergic cells from MPTP-induced damage. At present, the cellular mechanisms of these protective effects remain unclear, but it is tempting to speculate that they may be mediated in part by a reduction of intracytoplasmic calcium levels, which are normally increased by mGluR5-mediated activation of IP3 receptors (Pin and Duvoisin, 1995; Sala et al., 2005). There is, indeed, evidence that locus coeruleus neurons strongly express functional mGluR5, which are known to elicit excitatory effects and to potentiate N-methyl-d-aspartic acid-mediated excitation in these cells (Singewald et al., 1994, 1995, 1996; Page et al., 2005; Rasmussen et al., 2005, Noriega et al., 2007). As in the ventral midbrain, the potential downregulation of glutamatergic afferents, and the possible attenuation of inflammatory processes mediated by glial mGluR5 activation are other mechanisms that could explain the protective effects of MTEP in locus coeruleus/A5/A7 noradrenaline cell groups (see above).
Norepinephrine neurons are known to be involved in many autonomic and cognitive functions, and it is thought that dysfunction in cortical noradrenergic transmission contributes to depression (Remy et al., 2005; Arakawa et al., 2008; Samuels and Szabadi 2008; Lockrow et al., 2011), a common non-motor symptom in Parkinson’s disease (Picillo et al., 2009; Brooks and Pavese, 2010; Chaudhuri and Odin, 2010). The discovery of neuroprotective approaches that could attenuate norepinephrine cell loss in Parkinson’s disease would be highly interesting. The need for such preventive therapy is further strengthened by recent studies suggesting that norepinephrine brainstem neurons may die earlier than dopaminergic neurons in Parkinson’s disease, and that the loss of norepinephrine cells may even contribute to the later demise of substantia nigra pars compacta neurons (Fornai et al., 1997; Archer et al., 2006; Rommelfanger et al., 2007a, b).
Conclusion
The metabotropic glutamate receptors have become of great interest for the development of pharmacotherapeutic agents in various brain diseases. Their allosteric modulatory sites combined with their selective pharmacology and specific enrichment in particular brain areas provide them the characteristics that overcome the limitations faced by ionotropic glutamate receptor antagonists when used as brain therapeutics (Conn et al., 2005; Marino and Conn, 2006; Johnson et al., 2009; Niswender and Conn, 2010). In light of recent rodent and monkey behavioural data, mGluR5 has been recognized as a highly relevant antiparkinsonian and antidyskinetic target (Breysse et al., 2002; Johnston et al., 2010; Morin et al., 2010; Rylander et al., 2010). These positive results are further amplified by the finding of neuroprotective effects in monkeys, as documented in our study. However, in light of recent failures in translating positive neuroprotective results gathered from animal model studies to successful clinical trials in patients with Parkinson’s disease (Olanow, 2009), our data must be interpreted cautiously. Although various issues were raised to explain these failures (Olanow, 2009), we believe that the translation of our findings to the human trial setting must await the development of biomarkers that will allow the pretreatment of potential candidates for the future development of Parkinson’s disease before the appearance of motor symptoms and significant degeneration of dopaminergic and noradrenergic neurons. While the currently available mGluR5 antagonists, MTEP and MPEP, may not be ideal clinical treatment candidates because of their rapid brain clearance (Anderson et al., 2003; Lea and Faden, 2006; Johnston et al., 2010; Niswender and Conn, 2010), the future development of new negative allosteric modulators of mGluR5 with a better pharmacokinetic profile may pave the way for future human trials (Homayoun et al., 2004; Berry-Kravis et al., 2009; Bird and Lawrence, 2009; Niswender and Conn, 2010; Rodriguez et al., 2010; Stefani and Moghaddam 2010).
Funding
A grant from the National Parkinson Foundation to Y.S. and the Yerkes National Primate Research Center base grant from the National Institute of Health (RR00165).
Acknowledgements
Thanks are due to Susan Jenkins and Jean-Francois Pare for technical assistance, Vasavi Reddy, Xing Hu and Adriana Galvan for training with monkey behaviour and Rosa Villalba for help with the use of stereology.
Glossary
Abbreviations
- FECNT
2β-carbomethoxy-3β-(4-chlorophenyl)-8-(2-[18F]-fluoroethyl)-nortropane
- mGluR5
metabotropic glutamate receptor 5
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MTEP
3-[(2-methyl-1,3-thiazol-4-yl) ethynyl]
- PET
positron emission tomography
References
- Aarsland D, Larsen JP, Lim NG, Janvin C, Karlsen K, Tandberg E, et al. Range of neuropsychiatric disturbances in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67:492–6. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Andersen K, Larsen JP, Perry R, Wentzel-Larsen T, Lolk A, et al. The rate of cognitive decline in Parkinson disease. Arch Neurol. 2004;61:1906–11. doi: 10.1001/archneur.61.12.1906. [DOI] [PubMed] [Google Scholar]
- Aguirre JA, Kehr J, Yoshitake T, Liu FL, Rivera A, Fernandez-Espinola S. Protection but maintained dysfunction of nigral dopaminergic nerve cell bodies and striatal dopaminergic terminals in MPTP-lesioned mice after acute treatment with the mGluR5 antagonist MPEP. Brain Res. 2005;1033:216–20. doi: 10.1016/j.brainres.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Alagarsamy S, Rouse ST, Junge C, Hubert GW, Gutman D, Smith Y, et al. NMDA induced phosphorylation and regulation of mGluR5. Pharmacol Biochem Behav. 2002;73:299–306. doi: 10.1016/s0091-3057(02)00826-2. [DOI] [PubMed] [Google Scholar]
- Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, et al. In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligant [3H}3-methoxy-5-(pyridine-2-ylethynyl)pyridine) Eur J Pharmacol. 2003;473:35–40. doi: 10.1016/s0014-2999(03)01935-6. [DOI] [PubMed] [Google Scholar]
- Arakawa R, Okumura M, Ito H, Seki C, Takahashi H, Takano H, et al. Quantitative analysis of norepinephrine transporter in the human brain using PET with (S,S)-18F-FMeNER-D2. J Nucl Med. 2008;49:1270–6. doi: 10.2967/jnumed.108.051292. [DOI] [PubMed] [Google Scholar]
- Archer T, Fredriksson A. Influence of noradrenaline denervation on MPTP-induced deficits in mice. J Neural Transm. 2006;113:1119–29. doi: 10.1007/s00702-005-0402-5. [DOI] [PubMed] [Google Scholar]
- Armentero MT, Fancellu R, Nappi G, Bramanti P, Blandini F. Prolonged blockade of NMDA or mGluR5 glutamate receptors reduces nigrostriatal degeneration while inducing selective metabolic changes in the basal ganglia circuitry in a rodent model of Parkinson’s disease. Neurobiol Dis. 2006;22:1–9. doi: 10.1016/j.nbd.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–9. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone P. Neurotransmission in Parkinson’s disease: beyond dopamine. Eur J Neurol. 2010;17:364–76. doi: 10.1111/j.1468-1331.2009.02900.x. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Busceti CL, Molinaro G, Biagioni F, Storto M, Fornai F, et al. Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigro-striatal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J Neurosci. 2004;24:828–35. doi: 10.1523/JNEUROSCI.3831-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology. 2009;73:1699–704. doi: 10.1212/WNL.0b013e3181c2937c. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, et al. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009;46:266–71. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Boraud T, Bioulac B, Gross CE. Involvement of the subthalamic nucleus in glutamatergic compensatory mechanisms. Eur J Neurosci. 1999;11:2167–70. doi: 10.1046/j.1460-9568.1999.00627.x. [DOI] [PubMed] [Google Scholar]
- Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, et al. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Neurosci. 2001;21:6853–61. doi: 10.1523/JNEUROSCI.21-17-06853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biglan KM, Ravina B. Neuroprotection in Parkinson’s disease: an elusive goal. [Review] Semin Neurol. 2007;27:106–12. doi: 10.1055/s-2007-971168. [DOI] [PubMed] [Google Scholar]
- Bird MK, Lawrence AJ. The promiscuous mGlu5 receptor–a range of partners for therapeutic possibilities? Trends Pharmacol Sci. 2009;30:617–23. doi: 10.1016/j.tips.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Bogenpohl JW, Verreault M, Galvan A, Liu J, Smith Y. The use of mGluR5 antagonists as antiparkinsonian therapy in MPTP-treated monkeys. SFN Abstr. 2006:P175.3. [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Breysse N, Amalric M, Salin P. Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basal-ganglia structures in parkinsonian rats. J Neurosci. 2003;23:8302–9. doi: 10.1523/JNEUROSCI.23-23-08302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breysse N, Baunez C, Spooren W, Gasparini F, Amalric M. Chronic but not acute treatment with a metabotropic glutamate 5 receptor antagonist reverses the akinetic deficits in a rat model of parkinsonism. J Neurosci. 2002;22:5669–78. doi: 10.1523/JNEUROSCI.22-13-05669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DJ, Pavese N. Imaging non-motor aspects of Parkinson’s disease. Prog Brain Res. 2010;184:205–18. doi: 10.1016/S0079-6123(10)84011-7. [DOI] [PubMed] [Google Scholar]
- Brouillet E, Beal MF. NMDA antagonists partially protect against MPTP-induced neurotoxicity in mice. Neuro Report. 1993;4:387–90. doi: 10.1097/00001756-199304000-00011. [DOI] [PubMed] [Google Scholar]
- Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci USA. 1983;80:4546–50. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L, et al. The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology. 2004;29:1971–9. doi: 10.1038/sj.npp.1300540. [DOI] [PubMed] [Google Scholar]
- Byrnes KR, Stoica B, Riccio A, Pajoohesh-Ganji A, Loane DJ, Faden AI. Activation of metabotropic glutamate receptor 5 improves recovery after spinal cord injury in rodents. Ann Neurol. 2009;66:63–74. doi: 10.1002/ana.21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Endogenous ACh enhances striatal NMDA responses via M1-like muscarinic receptors and PKC activation. Eur J Neurosci. 1998;10:2887–95. doi: 10.1111/j.1460-9568.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT. Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog Brain Res. 2010;184:17–33. doi: 10.1016/S0079-6123(10)84002-6. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Zhang J. Glutamate, excitotoxicity, and programmed cell death in Parkinson disease. Exp Neurol. 2009;220:230–3. doi: 10.1016/j.expneurol.2009.09.027. [DOI] [PubMed] [Google Scholar]
- Chan CS, Gertler TS, Surmeier DJ. Calcium homeostasis, selective vulnerability and Parkinson’s disease. Trends Neurosci. 2009;32:249–56. doi: 10.1016/j.tins.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V. Locus coeruleus and norepinephrine in Parkinson’s disease. Jpn J Psychiatry Neurol. 1991;45:519–21. doi: 10.1111/j.1440-1819.1991.tb02540.x. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Odin P. The challenge of non-motor symptoms in Parkinson’s disease. Prog Brain Res. 2010;184:325–41. doi: 10.1016/S0079-6123(10)84017-8. [DOI] [PubMed] [Google Scholar]
- Chen MK, Lee JS, McGlothan JL, Furukawa E, Adams RJ, Alexander M, et al. Acute manganese administration alters dopamine transporter levels in the non-human primate striatum. Neurotoxicology. 2006;27:229–36. doi: 10.1016/j.neuro.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Clarke CE. A “cure” for Parkinson’s disease: can neuroprotection be proven with current trial designs? [Review] Mov Disord. 2004;19:491–8. doi: 10.1002/mds.20057. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. [Review] Nat Rev Neurosci. 2005;6:787–98. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–26. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- Drouin-Ouellet J, Brownell AL, Saint-Pierre M, Fasano C, Emond V, Trudeau LE, et al. Neuroinflammation is associated with changes in glial mGluR5 expression and the development of neonatal excitotoxic lesions. Glia. 2011;59:188–99. doi: 10.1002/glia.21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazal A, Parker F, Palmer AM, Croucher MJ. Characterisation of the actions of group I metabotropic glutamate receptor subtype selective ligands on excitatory amino acid release and sodium-dependent re-uptake in rat cerebrocortical minislices. J Neurochem. 2003;86:1346–58. doi: 10.1046/j.1471-4159.2003.01932.x. [DOI] [PubMed] [Google Scholar]
- Fornai F, Bassi L, Torracca MT, Scalori V, Corsini GU. Norepinephrine loss exacerbates methamphetamine-induced striatal dopamine depletion in mice. Eur J Pharmacol. 1995;283:99–102. doi: 10.1016/0014-2999(95)00313-a. [DOI] [PubMed] [Google Scholar]
- Fornai F, Alessandrì MG, Torracca MT, Bassi L, Corsini GU. Effects of noradrenergic lesions on MPTP/MPP+ kinetics and MPTP-induced nigrostriatal dopamine depletions. J Pharmacol Exp Ther. 1997;283:100–7. [PubMed] [Google Scholar]
- Fornai F, di Poggio AB, Pellegrini A, Ruggieri S, Paparelli A. Noradrenaline in Parkinson’s disease: from disease progression to current therapeutics. Curr Med Chem. 2007;14:2330–4. doi: 10.2174/092986707781745550. [DOI] [PubMed] [Google Scholar]
- Fornai F, Schlüter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, et al. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci USA. 2005;102:3413–8. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno LS, Langston JW, DeLanney LE, Irwin I, Ricaurte GA. Locus ceruleus lesions and eosinophilic inclusions in MPTP-treated monkeys. Ann Neurol. 1986;20:449–55. doi: 10.1002/ana.410200403. [DOI] [PubMed] [Google Scholar]
- Forno LS, DeLanney LE, Irwin I, Langston JW. Similarities and differences between MPTP-induced parkinsonism and Parkinson’s disease. Neuropathologic considerations. Adv Neurol. 1993;60:600–8. [PubMed] [Google Scholar]
- Frisina PG, Haroutunian V, Libow LS. The neuropathological basis for depression in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:144–8. doi: 10.1016/j.parkreldis.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Bilbe G, Gomez-Mancilla B, Spooren W. mGluR5 antagonists: discovery, characterization and drug development. Curr Opin Drug Discov Devel. 2008;11:655–65. [PubMed] [Google Scholar]
- German DC, Manaye KF, White CL, 3rd, Woodward DJ, McIntire DD, Smith WK, et al. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32:667–76. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Terruli M, Lees AJ, Jenner P, Marsden CD. The evolution and distribution of morphological changes in the nervous system of the common marmoset following the acute administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Mov Disord. 1989;4:53–74. doi: 10.1002/mds.870040109. [DOI] [PubMed] [Google Scholar]
- Gillette JS, Bloomquist JR. Differential up-regulation of striatal dopamine transporter and α-synuclein by the pyrethroid insecticide permethrin. Toxicol Appl Pharmacol. 2003;192:287–93. doi: 10.1016/s0041-008x(03)00326-0. [DOI] [PubMed] [Google Scholar]
- Grimbergen YAM, Munneke M, Bloem BR. Falls in Parkinson’s disease. Curr Opin Neurol. 2004;17:405–15. doi: 10.1097/01.wco.0000137530.68867.93. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RG, Howe PR, et al. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol. 1990;27:373–85. doi: 10.1002/ana.410270405. [DOI] [PubMed] [Google Scholar]
- Herrero MT, Hirsch EC, Javoy-Agid F, Obeso JA, Agid Y. Differential vulnerability to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine of dopaminergic and cholinergic neurons in the monkey mesopontine tegmentum. Brain Res. 1993;624:281–5. doi: 10.1016/0006-8993(93)90088-5. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology. 2004;29:1259–69. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Horowitz MP, Greenamyre JT. Gene-environment interactions in Parkinson’s disease: the importance of animal modeling. Clin Pharmacol Ther. 2010;88:467–74. doi: 10.1038/clpt.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–80. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Paquet M, Smith Y. Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey substantia nigra. 2001;21:1838–47. doi: 10.1523/JNEUROSCI.21-06-01838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert GW, Smith Y. Age-related changes in the expression of axonal and glial group I metabotropic glutamate receptor in the rat substantia nigra pars reticulata. J Comp Neurol. 2004;475:95–106. doi: 10.1002/cne.20163. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Conn PJ, Niswender CM. Glutamate receptors as therapeutic targets for Parkinson's disease. CNS Neurol Disord Drug Targets. 2009;8:475–91. doi: 10.2174/187152709789824606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston TH, Fox SH, McIldowie MJ, Piggott MJ, Brotchie JM. Reduction of L-DOPA induced dyskinesia by the selective metabotropic glutamate receptor 5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Pharmacol Exp Ther. 2010;333:865–73. doi: 10.1124/jpet.110.166629. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kanthasamy A, Matsumoto RR, Vu TQ, Truong DD. Neuroprotective effects of the strychnine-insensitive glycine site NMDA antagonist (R)-HA-966 in an experimental model of Parkinson’s disease. Brain Res. 1997;759:1–8. doi: 10.1016/s0006-8993(96)01192-4. [DOI] [PubMed] [Google Scholar]
- Kieburtz K, Ravina B. Why hasn’t neuroprotection worked in Parkinson’s disease? [Review] Nat Clin Pract Neurol. 2007;3:240–1. doi: 10.1038/ncpneuro0491. [DOI] [PubMed] [Google Scholar]
- Kim WT, Rioult MG, Cornell-Bell AH. Glutamate-induced calcium signaling in astrocytes. Glia. 1994;11:173–84. doi: 10.1002/glia.440110211. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Barlow RL, Bloomquist JR. Neurotoxicity of the organochlorine insecticide heptachlor to murine striatal dopaminergic pathways. Toxicol Sci. 2001;61:100–6. doi: 10.1093/toxsci/61.1.100. [DOI] [PubMed] [Google Scholar]
- Konitsiotis S, Blanchet PJ, Verhagen L, Lamers E, Chase TN. AMPA receptor blockade improves levodopa-induced dyskinesia in MPTP monkeys. Neurology. 2000;54:1589–95. doi: 10.1212/wnl.54.8.1589. [DOI] [PubMed] [Google Scholar]
- Lange KW, Kornhuber J, Riederer P. Dopamine/glutamate interactions in Parkinson’s disease. Neurosci Biobehav Rev. 1997;21:393–400. doi: 10.1016/s0149-7634(96)00043-7. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Rebert CS, Irwin I. Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyrine (MPTP) in the squirrel monkey. Brain Res. 1984;292:390–4. doi: 10.1016/0006-8993(84)90777-7. [DOI] [PubMed] [Google Scholar]
- Langston JW. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol. 2006;59:591–6. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- Lea PMt, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12:149–66. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandis G, Bazzini E, Armentero MT, Nappi G, Blandini F. Systemic administration of an mGluR5 antagonist, but not unilateral subthalamic lesion, counteracts l-DOPA-induced dyskinesias in a rodent model of Parkinson’s disease. Neurobiol Dis. 2008;29:161–8. doi: 10.1016/j.nbd.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Stoica BA, Pajoohesh-Ganji A, Byrnes KR, Faden AI. Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase. J Biol Chem. 2009;584:15629–39. doi: 10.1074/jbc.M806139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockrow J, Boger H, Gerhardt G, Bachman D, Granholm AC. A noradrenergic lesion exacerbates neurodegeneration in a down syndrome mouse model. J Alzheimers Dis. 2011;23:471–89. doi: 10.3233/JAD-2010-101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschmann PA, Lange KW, Wachtel H, Turski L. MPTP-induced degeneration: interference with glutamatergic toxicity. J Neural Transm. 1994;(Suppl 43):133–43. [PubMed] [Google Scholar]
- Maesawa S, Kaneoke Y, Kajita Y, Usui N, Misawa N, Nakayama A, et al. Long-term stimulation of the subthalamic nucleus in hemiparkinsonian rats: neuroprotection of dopaminergic neurons. J Neurosurg. 2004;100:679–87. doi: 10.3171/jns.2004.100.4.0679. [DOI] [PubMed] [Google Scholar]
- Marien M, Briley M, Colpaert F. Noradrenaline depletion exacerbates MPTP-induced striatal dopamine loss in mice. Eur J Pharmacol. 1993;236:487–9. doi: 10.1016/0014-2999(93)90489-5. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Conn PJ. Glutamate-based therapeutic approaches: allosteric modulators of metabotropic glutamate receptors [Review] Curr Opin Pharmacol. 2006;6:98–102. doi: 10.1016/j.coph.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Valenti O, Conn PJ. Glutamate receptors and Parkinson’s disease: opportunities for intervention. Drugs Aging. 2003;20:377–97. doi: 10.2165/00002512-200320050-00006. [DOI] [PubMed] [Google Scholar]
- Masilamoni JG, Alagille D, Bogenpohl J, Delevich K, Reddy V, Votaw JR, et al. Potential neuroprotective effects of metabotropic glutamate receptor 5 antagonist in MPTP-treated Parkinsonian monkeys. SFN Abstr. 2009:P 828.17. [Google Scholar]
- Masilamoni G, Votaw J, Howell L, Villalba RM, Goodman M, Voll RJ, et al. 18F-FECNT: validation as PET dopamine transporter ligand in Parkinsonism. Exp Neurol. 2010a;226:265–73. doi: 10.1016/j.expneurol.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masilamoni JG, Alagille D, Jenkins S, Tamagnan G, Wichmann T, Smith Y. Metabotropic glutamate receptor 5 antagonist protect dopaminergic and noradrenergic neurons against MPTP neurotoxin in parkinsonian monkey model. SFN Abstr. 2010b:P460.5. [Google Scholar]
- Mitchell IJ, Cross AJ, Sambrook MA, Crossman AR. Sites of the neurotoxic action of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the macaque monkey include the ventral tegmental area and the locus coeruleus. Neurosci Lett. 1985;61:195–200. doi: 10.1016/0304-3940(85)90424-0. [DOI] [PubMed] [Google Scholar]
- Miyoshi R, Kito S, Ishida H, Katayama S. Alterations of the central noradrenergic system in MPTP-induced monkey parkinsonism. Res Commun Chem Pathol Pharmacol. 1988;62:93–102. [PubMed] [Google Scholar]
- Morin N, Grégoire L, Gomez-Mancilla B, Gasparini F, Di Paolo T. Effect of the metabotropic glutamate receptor type 5 antagonists MPEP and MTEP in parkinsonian monkeys. Neuropharmacology. 2010;58:981–6. doi: 10.1016/j.neuropharm.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol. 2006;6:53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Nakajima Y, Masu M, Ueda Y, Nakahara K, Watanabe D, et al. Glutamate receptors: brain function and signal transduction. Brain Res Rev. 1998;26:230–5. doi: 10.1016/s0165-0173(97)00033-7. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, et al. Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology. 2010;XX:1–25. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease [Review] Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega NC, Garyfallou VT, Kohama SG, Urbanski HF. Glutamate receptor subunit expression in the rhesus macaque locus coeruleus. Brain Res. 2007;1173:53–65. doi: 10.1016/j.brainres.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW. Can we achieve neuroprotection with currently available anti-parkinsonian interventions? [Review] Neurology. 2009;72(Suppl 7):S59–64. doi: 10.1212/WNL.0b013e318199068b. [DOI] [PubMed] [Google Scholar]
- Ossowska K, Konieczny J, Wardas J, Pietraszek M, Kuter K, Wolfarth S, et al. An influence of ligands of metabotropic glutamate receptor subtypes on parkinsonian-like symptoms and the striatopallidal pathway in rats. Amino Acids. 2007;32:179–88. doi: 10.1007/s00726-006-0317-y. [DOI] [PubMed] [Google Scholar]
- Ossowska K. The role of excitatory amino acids in experimental models of Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1994;8:39–71. doi: 10.1007/BF02250917. [DOI] [PubMed] [Google Scholar]
- Page ME, Szeliga P, Gasparini F, Cryan JF. Blockade of the mGlu5 receptor decreases basal and stress-induced cortical norepinephrine in rodents. Psychopharmacology (Berl) 2005;179:240–6. doi: 10.1007/s00213-005-2142-5. [DOI] [PubMed] [Google Scholar]
- Patt S, Gerhard L. A Golgi study of human locus coeruleus in normal brains and in Parkinson’s disease. Neuropathol Appl Neurobiol. 1993;19:519–23. doi: 10.1111/j.1365-2990.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Pavese N, Kiferle L, Piccini P. Neuroprotection and imaging studies in Parkinson’s disease. [Review] Parkinsonism Relat Disord. 2009;15(Suppl 4):S33–7. doi: 10.1016/S1353-8020(09)70832-6. [DOI] [PubMed] [Google Scholar]
- Pavese N, Brooks DJ. Imaging neurodegeneration in Parkinson’s disease. [Review] Biochim Biophys Acta. 2009;1792:722–9. doi: 10.1016/j.bbadis.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Lam HA, Ackerson LC, Maidment NT. Blockade of mGluR glutamate receptors in the subthalamic nucleus ameliorates motor asymmetry in an animal model of Parkinson’s disease. Eur J Neurosci. 2006;23:151–60. doi: 10.1111/j.1460-9568.2005.04550.x. [DOI] [PubMed] [Google Scholar]
- Piallat B, Polosan M, Fraix V, Goetz L, David O, Fenoy A, et al. Subthalamic neuronal firing in obsessive-compulsive disorder and Parkinson disease. Ann Neurol. 2011;69:793–802. doi: 10.1002/ana.22222. [DOI] [PubMed] [Google Scholar]
- Picillo M, Rocco M, Barone P. Dopamine receptor agonists and depression in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 4):S81–4. doi: 10.1016/S1353-8020(09)70841-7. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. [Review] Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Martin H, Berger JE, Seager MA. The mGlu5 receptor antagonists MPEP and MTEP attenuate behavioral signs of morphine withdrawal and morphine-withdrawal-induced activation of locus coeruleus neurons in rats. Neuropharmacology. 2005;48:173–80. doi: 10.1016/j.neuropharm.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314–22. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- Rodriguez AL, Grier MD, Jones CK, Herman EJ, Kane AS, Smith RL, et al. Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity. Mol Pharmacol. 2010;78:1105–23. doi: 10.1124/mol.110.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MC, Guridi OJ, Alvarez L, Mewes K, Macias R, Vitek J, et al. The subthalamic nucleus and tremor in Parkinson’s disease. [Review] Mov Disord. 1998;13(Suppl 3):111–8. doi: 10.1002/mds.870131320. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D. Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci USA. 2007a;104:13804–9. doi: 10.1073/pnas.0702753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: the redheaded stepchild of Parkinson’s disease. [Review] Biochem Pharmacol. 2007b;74:177–90. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Rose S, Nomoto M, Jackson EA, Gibb WR, Jaehnig P, Jenner P, et al. Age-related effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment of common marmosets. Eur J Pharmacol. 1993;230:177–85. doi: 10.1016/0014-2999(93)90800-w. [DOI] [PubMed] [Google Scholar]
- Rylander D, Iderberg H, Li Q, Dekundy A, Zhang J, Li H, et al. A mGluR5 antagonist under clinical development improves L-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol Dis. 2010;39:352–61. doi: 10.1016/j.nbd.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Rylander D, Recchia A, Mela F, Dekundy A, Danysz W, Cenci MA. Pharmacological modulation of glutamate transmission in a rat model of L-DOPA-induced dyskinesia: effects on motor behavior and striatal nuclear signaling. J Pharmacol Exp Ther. 2009;330:227–35. doi: 10.1124/jpet.108.150425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Roussignol G, Meldolesi J, Fagni L. Key role of the postsynaptic density scaffold proteins Shank and Homer in the functional architecture of Ca2+ homeostasis at dendritic spines in hippocampal neurons. J Neurosci. 2005;25:4587–92. doi: 10.1523/JNEUROSCI.4822-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol. 2008;6:254–85. doi: 10.2174/157015908785777193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Olanow CW. Neuroprotection in Parkinson disease: mysteries, myths, and misconceptions. JAMA. 2004;291:358–64. doi: 10.1001/jama.291.3.358. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Progress in neuroprotection in Parkinson’s disease. [Review] Eur J Neurol. 2008;15(Suppl 1):5–13. doi: 10.1111/j.1468-1331.2008.02055.x. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Greenamyre JT. Environment, mitochondria, and Parkinson’s disease. Neuroscientist. 2002;8:192–7. doi: 10.1177/1073858402008003004. [DOI] [PubMed] [Google Scholar]
- Shimo Y, Wichmann T. Neuronal activity in the subthalamic nucleus modulates the release of dopamine in the monkey striatum. [Review] Eur J Neurosci. 2009;29:104–13. doi: 10.1111/j.1460-9568.2008.06565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Schneider C, Philippu A. Effects of neuroactive compounds, noxious and cardiovascular stimuli on the release of amino acids in the rat locus coeruleus. Neurosci Lett. 1994;180:55–8. doi: 10.1016/0304-3940(94)90912-1. [DOI] [PubMed] [Google Scholar]
- Singewald N, Zhou GY, Chen F, Philippu A. Corticotropin-releasing factor modulates basal and stress-induced excitatory amino acid release in the locus coeruleus of conscious rats. Neurosci Lett. 1996;204:45–8. doi: 10.1016/0304-3940(96)12312-0. [DOI] [PubMed] [Google Scholar]
- Singewald N, Zhou GY, Schneider C. Release of excitatory and inhibitory amino acids from the locus coeruleus of conscious rats by cardiovascular stimuli and various forms of acute stress. Brain Res. 1995;704:42–50. doi: 10.1016/0006-8993(95)01102-1. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. Convergence of synaptic inputs from the striatum and the globus pallidus onto identified nigrocollicular cells in the rat: a double anterograde labelling study. Neuroscience. 1991;44:45–73. doi: 10.1016/0306-4522(91)90250-r. [DOI] [PubMed] [Google Scholar]
- Song DD, Haber SN. Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J Neurosci. 2000;20:5102–14. doi: 10.1523/JNEUROSCI.20-13-05102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonsalla PK, Albers DS, Zeevalk GD. Role of glutamate in neurodegeneration of dopamine neurons in several animal models of parkinsonism. Amino Acids. 1998;14:69–74. doi: 10.1007/BF01345245. [DOI] [PubMed] [Google Scholar]
- Srivastava R, Brouillet E, Beal MF, Storey E, Hyman BT. Blockade of 1-methyl-4-phenylpyridinium ion (MPP+) nigral toxicity in the rat by prior decortication or MK-801 treatment: a stereological estimate of neuronal loss. Neurobiol Aging. 1993;14:295–301. doi: 10.1016/0197-4580(93)90114-q. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. Eur J Pharm. 2010;639:26–32. doi: 10.1016/j.ejphar.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–92. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Turski L, Bressler K, Rettig KJ, Löschmann PA, Wachtel H. Protection of substantia nigra from MPP+ neurotoxicity by N-methyl-D-aspartate antagonists. Nature. 1991;349:414–8. doi: 10.1038/349414a0. [DOI] [PubMed] [Google Scholar]
- Van Laar VS, Berman SB. Mitochondrial dynamics in Parkinson’s disease. Biochim Biophys Acta. 2010;1802:29–44. doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Vernon AC, Zbarsky V, Datla KP, Croucher MJ, Dexter DT. Subtype selective antagonism of substantia nigra pars compacta Group I metabotropic glutamate receptors protects the nigrostriatal system against 6-hydroxydopamine toxicity in vivo. J Neurochem. 2007;103:1075–91. doi: 10.1111/j.1471-4159.2007.04860.x. [DOI] [PubMed] [Google Scholar]
- Votaw JR, Howell LL, Martarello L, Hoffman JM, Kilts CD, Lindsey KP, et al. Measurement of dopamine transporter occupancy for multiple injections of cocaine using a single injection of [F-18]FECNT. Synapse. 2002;44:203–10. doi: 10.1002/syn.10068. [DOI] [PubMed] [Google Scholar]
- Wallace BA, Ashkan K, Heise CE, Foote KD, Torres N, Mitrofanis J, et al. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain. 2007;130:2129–45. doi: 10.1093/brain/awm137. [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Exp Neurol. 2009;218:247–56. [Google Scholar]
- Yacoubian TA, Standaert DG. Targets for neuroprotection in Parkinson’s disease. [Review] Biochim Biophys Acta. 2009;1792:676–87. doi: 10.1016/j.bbadis.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarow C, Lyness SA, Mortimer JA, Chui HC. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–41. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- Zesiewicz TA, Baker MJ, Wahba M, Hauser RA. Autonomic nervous system dysfunction in Parkinson’s disease. Curr Treat Options Neurol. 2003;5:149–60. doi: 10.1007/s11940-003-0005-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rodriguez AL, Conn PJ. Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J Pharmacol Exp Ther. 2005;315:1212–9. doi: 10.1124/jpet.105.090308. [DOI] [PubMed] [Google Scholar]