Abstract
Danoprevir is a potent and selective direct-acting antiviral agent that targets the protease activity of hepatitis C virus (HCV) NS3/4A. This agent results in a significant rapid decline in HCV RNA levels when it is used in monotherapy. The present study evaluated whether plasma concentrations of the inflammatory markers gamma interferon-inducible protein 10 (IP-10) and neopterin or the interferon-stimulated gene product 2′-5′-oligoadenylate synthetase (OAS-1) were correlated with the plasma HCV RNA concentration before or during 14-day danoprevir monotherapy. In contrast to pegylated interferon and ribavirin treatment, a higher baseline IP-10 concentration was positively correlated with a greater first-phase HCV RNA decline upon danoprevir administration. Changes in the IP-10 plasma concentration during danoprevir administration were also associated with categorical changes in HCV RNA concentration at days 7 and 14. The neopterin concentration appeared to be moderately decreased during danoprevir administration, although these changes were not statistically significant. However, changes in neopterin concentration showed a statistically significant correlation with changes in IP-10 concentration. Considerable variation in the OAS-1 concentration was observed before and during treatment, including in patients treated with placebo and/or patients with minimal virologic response. Overall, these results suggest that effective treatment with a direct-acting antiviral agent may reduce hepatic inflammation and that first-phase HCV RNA decline during treatment with an NS3/4A protease inhibitor is more robust in patients with high baseline IP-10 concentrations.
INTRODUCTION
Hepatitis C virus (HCV) is a positive-strand RNA virus belonging to the Flaviviridae family. Approximately 170 million people worldwide suffer from chronic HCV infection (2, 47), which is a major cause of chronic liver disease, cirrhosis, and primary hepatocellular carcinoma and is currently among the leading indications for liver transplantation in both the United States and Europe (1, 45). Progression to liver cirrhosis/fibrosis may be asymptomatic and can evolve over many years (4). The current standard of care (SOC) for chronic HCV infection is a 24- to 48-week regimen comprised of weekly subcutaneous injections of pegylated alpha interferon (PEG-IFN) and twice daily (BID) oral ribavirin treatment. This regimen leads to a sustained virologic response (SVR) in approximately half of the patients infected with HCV genotype 1 (5, 13).
Chronic HCV infection is characterized by persistent inflammation of hepatic lobules and the presence of CXCR3-postive T cells (21, 56). The extent of cellular infiltration correlates with stage of disease (54, 55). The majority of these cells are not HCV specific and not competent to clear virus (28). However, they contribute to a persistent inflammatory process which is thought to contribute to cell death, liver damage, and the advent and progression of hepatic fibrosis (28, 56).
Biomarkers that predict antiviral treatment response or correlate with the extent of ongoing inflammation or fibrosis would be useful to monitor the disease state in patients with chronic HCV infection. Several biomarkers have recently been evaluated in the context of HCV infection, including interferon-responsive genes (26, 30, 37, 40, 51, 53), chemokines (28, 29, 31, 54, 56), interleukin-28B (IL-28B) gene polymorphisms (16, 18, 39), fibrosis protein markers (6, 7, 35), fibrinogen-like protein 2 (11), complement component C3a (25), and osteopontin (23).
Several of the biomarkers evaluated in patients with HCV infection are associated with an interferon-mediated inflammatory response. One such biomarker is gamma interferon (IFN-γ)-inducible protein 10 (IP-10 or CXCL10), a chemokine produced by a variety of cells, including hepatocytes in areas of liver inflammation (21). IP-10 is a chemoattractant for CXCR3 receptor-expressing cells, including monocytes, natural killer cells, and T cells, and has been proposed to recruit activated T cells to hepatic lesions in chronic viral hepatitis (34, 41, 49, 55).
Patients with chronic HCV infection display elevated plasma concentrations of IP-10 (5, 8, 27, 33) which correlate with the degree of liver inflammation and fibrosis (28, 42, 54, 55). Elevated IP-10 mRNA expression in HCV-infected liver tissue also correlates with the accumulation of CXCR3-expressing T cells in the liver as well as histological fibrosis scores (21). Notably, a low baseline IP-10 concentration predicts a robust first-phase decline in HCV RNA in response to SOC (3), and the plasma IP-10 concentration is inversely correlated with rates of SVR (3, 5, 8, 9, 27).
Neopterin is associated with activation of the cellular immune system and is used as a marker of inflammation. Neopterin is released by monocytes/macrophages after activation by IFN-γ, and its concentration is elevated in several disease states, including bacterial and viral infections and autoimmune disorders (20, 24, 36, 52). Neopterin plasma concentrations are increased during HCV infection (10, 17, 19, 36, 52).
2′-5′-Oligoadenylate synthetase (OAS-1) is an interferon-stimulated gene product that is responsible for activation of the RNase L pathway in response to viral RNA. OAS-1 binds to double-stranded RNA and catalyzes the formation of 2′-5′-linked oligoadenylate, which in turn activates RNase L, an endogenous protein that degrades viral RNA, thereby inhibiting viral protein synthesis and replication (26, 32, 48). Several studies have reported increased OAS-1 expression during chronic HCV infection (26, 38, 48). Similar to the case with IP-10 (3, 5, 8, 9, 27), the baseline OAS-1 concentration is higher in nonresponders (NRs) to SOC treatment than in those that achieve SVR (48).
Danoprevir (ITMN191/RG7227) is a highly potent and selective macrocyclic, peptidomimetic inhibitor of the HCV NS3/4A serine protease (46). Interim analysis of a phase 2b program in combination with SOC demonstrated rapid virologic response (RVR) and early virologic response (EVR) rates of up to 86% and 92%, respectively (50). In a first-of-its-kind study combining two orally active direct-acting antiviral agents in patients with chronic HCV infection, treatment with the combination of danoprevir and the NS5B polymerase inhibitor mericitabine (RG7128) for 13 days resulted in up to 62% (5/8) of patients having HCV loads below the limit of detection (15). Danoprevir is currently in clinical development as a ritonavir-boosted agent in both types of regimens.
In early-stage clinical trials, danoprevir monotherapy resulted in rapid, dose-dependent reductions in HCV RNA levels. When danoprevir was administered for 14 days as monotherapy, the best-performing treatment-naïve (TN) cohort experienced median maximal and median end-of-treatment (EOT) viral load reductions of −3.9 and −3.8 log10 IU/ml, respectively. A single cohort of NRs to prior SOC treatment experienced more muted changes on these endpoints of −2.9 and −2.5 log10 IU/ml, respectively. The overall rate of viral rebound was low (10/37) and similar in TN (8/29) and NR (2/8) cohorts. Median viral response profiles for continuous-decline and rebound patients showed nearly coincident median viral response profiles through day 7 that diverged thereafter, suggesting relatively late emergence of viral resistance (44).
The current study examined plasma concentrations of IP-10, neopterin, and OAS-1 in samples collected during the above-mentioned 14-day multiple-ascending-dose study of danoprevir monotherapy in patients chronically infected with HCV genotype 1. IP-10 and neopterin were examined, as they are associated with HCV-induced liver inflammation. As a prototypical interferon-stimulated gene, OAS-1 was used to examine the hypothesis that NS3/4A can dampen viral sensing and interferon production (14, 43).
MATERIALS AND METHODS
Danoprevir monotherapy phase 1 clinical trial.
Details of a 14-day, randomized, double-blind, placebo-controlled study evaluating danoprevir monotherapy in patients chronically infected with HCV genotype 1 have recently been published (12). The first part of this study evaluated multiple ascending doses of danoprevir in treatment-naïve patients. Patients in this portion of the study received oral danoprevir at doses of 100 mg BID (n = 8), 100 mg three times day (TID) (n = 8), 200 mg BID (n = 5), or 200 mg TID (n = 8) or placebo equivalent (n = 8). Fewer patients were in the 200-mg BID cohort, as three patients were incorrectly dosed and excluded from all data analyses (including those of viral kinetics and biomarkers). The second part of the study examined a single dose level (300 mg BID; n = 8) or placebo (n = 2) in a cohort of nonresponders to prior PEG-IFN–ribavirin therapy. Of the patients described above, 45/47 were included in the biomarker analysis. One patient (receiving 100 mg BID) was excluded from biomarker analysis due to the lack of baseline biomarker data. A second excluded patient (a TN patient receiving placebo) was omitted due to the lack of viral load determination at baseline.
The clinical study was conducted in full accordance with the 1996 Declaration of Helsinki. The study protocol was reviewed and approved by the independent ethics committee at each participating clinical research facility, and written informed consent was obtained from each patient or legal guardian prior to screening for study participation.
Viral kinetics and virologic fate.
The antiviral activity of danoprevir was assessed by measuring plasma HCV RNA at scheduled intervals from baseline to day 14 using a Cobas Ampliprep/Cobas TaqMan HCV assay (Roche Molecular Diagnostics, Pleasanton, CA). Viral kinetics were calculated and represented as the median log change from baseline (day 0). Virologic response patterns (rebound, plateau, continuous decline) were determined in danoprevir-treated patients by comparison of the EOT viral load relative to the nadir viral load using the following definitions. Rebound patients (n = 10) were defined as those with an increase from the nadir of ≥1.0 log10 IU/ml. Plateau patients (n = 13) were defined as those with an increase from the nadir of <1.0 log10 IU/ml. All other patients were classified as having a continuous decline (n = 14).
Biomarker analysis.
Biomarkers were assessed in plasma samples collected prior to treatment on day 0 (baseline sample) and on day 7 and day 14.
Plasma IP-10 concentration.
Plasma IP-10 concentration was measured in duplicate at Southern Research (Birmingham, AL) using a Quantikine human CXCL10/IP-10 immunoassay kit (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions. Average values were reported as picrograms per milliliter. On the basis of the manufacturer's specifications, the mean minimal detection limit of this kit is 1.7 pg/ml (range, 0.4 to 4.5 pg/ml).
Plasma neopterin concentration.
Plasma neopterin concentration was measured in duplicate at Southern Research (Birmingham, AL) using human neopterin enzyme immunoassay kits purchased from Alpco Diagnostics (Salem, NH), according to the manufacturer's instructions. Average values were reported as nanomoles per liter (nM). On the basis of the manufacturer's specifications, the assay has a range of 2 to 250 nM.
Plasma OAS-1 concentration.
Plasma OAS-1 levels were measured in triplicate at Southern Research (Birmingham AL) using human 2′-5′-oligoadenyl-5′-triphosphate (2-5A) radioimmunoassay (RIA) kits (Eiken Chemical Company, Tokyo, Japan), according to the manufacturer's instructions. Briefly, OAS-1 in the samples was bound to a poly(I)·poly(C) agarose gel, and the bound complex was then separated from other serum components by centrifugation. The bound, partially purified OAS-1 was allowed to catalyze the production of 2-5A. The amount of 2-5A produced, which is proportional to the OAS-1 enzyme activity, was quantified in a competitive RIA using 125I-labeled 2-5A. In this assay, newly formed 2-5A competed with 125I-labeled 2-5A for the binding sites on the anti-2-5A antibody. The antibody-bound 125I-labeled 2-5A was separated from unbound label by centrifugation, and bound radioactivity was quantified using a Packard Cobra 5005 gamma counter (GMI, Ramsey, MN). Average values were reported as picomoles per liter (pM). On the basis of the manufacturer's specifications, the kit has a range of 100 to 8,100 pM.
Statistical analysis.
Statistical comparisons between groups were performed by 1-way analysis of variance (ANOVA), followed by Dunnett's multiple comparison test, using either Prism software (GraphPad Software, La Jolla, CA) or JMP7 software (SAS Institute, Inc., Cary, NC). Correlations between continuous variables were assessed by linear regression using GraphPad Prism software.
RESULTS
IP-10 concentrations.
At baseline, the median IP-10 plasma concentration for all patients was 421 pg/ml (see Fig. S1A in the supplemental material). Significant interpatient variability was noted, with a range of 113 to 4,004 pg/ml (35-fold). TN and NR patients showed median IP-10 values of 344 and 526 pg/ml, respectively. Baseline IP-10 concentrations of 474, 338, and 787 pg/ml were observed in patients that experienced virologic continuous decline, plateau, and rebound, respectively (see Fig. S1B in the supplemental material). The range of IP-10 values in TN patients and NRs and in the continuous-decline, plateau, and rebound groups overlapped significantly (see Fig. S1A and B, respectively, in the supplemental material). Baseline values of IP-10 were not correlated with baseline HCV RNA concentrations across all patients (r2 < 107, P = 0.99), in TN patients (r2 = 0.00043, P = 0.91), or in NRs (r2 = 0.14, P = 0.28) (see Fig. S1C in the supplemental material).
Interestingly, a significant positive correlation was observed between baseline IP-10 plasma concentration and the magnitude of the first phase of HCV RNA decline (defined as the reduction observed 24 h after initiation of therapy; r2 = 0.29, P = 0.0008; Fig. 1). In contrast, no correlation was observed between baseline IP-10 concentration and HCV RNA concentration at EOT (r2 = 0.01, P = 0.50) or during the second phase of HCV RNA kinetics (defined as an HCV RNA concentration change between day 3 and EOT; r2 = 0.05, P = 0.21).
Fig. 1.
First-phase decline in HCV RNA versus baseline plasma IP-10 concentration. A linear fit of first-phase decline in HCV RNA versus baseline IP-10 concentration shows a positive correlation (all patients, r2 = 0.29 and P = 0.0008). First-phase decline was defined as the reduction in HCV RNA observed 24 h after initiation of danoprevir treatment.
Changes in IP-10 plasma concentration were also associated with categorical changes in HCV RNA concentration upon danoprevir administration (Fig. 2). At day 7 and day 14, patients that displayed a 1- to 2-log10-IU/ml reduction in HCV RNA had median reductions in IP-10 of 46% and 43%, respectively (Fig. 2A and B, respectively). Patients with greater changes in HCV RNA concentration experienced marginally increased changes in IP-10 concentration; a 4- to 5-log10-IU/ml reduction in HCV RNA was associated with median reductions in IP-10 of 65% at both day 7 and day 14 (Fig. 2A and B, respectively). In contrast, patients that experienced a <1-log10-IU/ml reduction in HCV RNA (mostly placebo-treated patients) showed a more stable median IP-10 concentration at both time points (Fig. 2A and B). The association of changes in the concentrations of HCV RNA and IP-10 was similar in TN patients and NRs (compare circles and triangles in Fig. 2A and B).
Fig. 2.
Change in plasma IP-10 concentration relative to categorical change in HCV RNA load. The percent change from baseline for individual patients at day 7 (A) or day 14 (B) is shown for five categories of change in HCV RNA. Medians and interquartile ranges are shown. Placebo-treated patients are shown as open black circles (TN patients) and open gray triangles (NRs). Danoprevir-treated patients are shown as closed black circles (TN patients) and closed gray triangles (NRs). Asterisks denote groups that show statistically significant differences relative to those experiencing a negligible (0- to 1-log10 IU/ml) change in viral load (P < 0.05; 1-way ANOVA followed by Dunnett's test).
The dynamics of IP-10 plasma concentration changes were distinct in patients experiencing a virologic continuous decline, plateau, or rebound during danoprevir administration (Fig. 3 A to C, respectively). Continuous-decline patients showed strong first- and second-phase median HCV RNA declines, with large declines in median IP-10 concentration (Fig. 3A). Continuous-decline patients experienced median reductions of baseline IP-10 concentration of 57% and 60% at day 7 and day 14, respectively. All but one continuous-decline patient had a lower plasma IP-10 concentration at day 14 relative to that at baseline; the remaining patient experienced a 6% increase from a low baseline concentration (160 pg/ml). Plateau patients experienced a lesser first-phase HCV RNA decline without evidence of second-phase HCV RNA decline through day 14. The corresponding median IP-10 concentrations in this group were similarly reduced at day 7 and day 14 by 43% and 39%, respectively (Fig. 3B). All but one plateau patient had a lower plasma IP-10 concentration at day 14 relative to that at baseline; the remaining patient experienced a 10% increase from a relatively low baseline concentration (190 pg/ml). Rebound patients showed a robust first-phase decline in HCV RNA concentration and a median decrease in plasma IP-10 concentration of 55% at day 7. The median IP-10 concentration at day 14 was reduced 71% relative to that at baseline. However, 3/9 rebound patients displayed considerably higher IP-10 concentrations at day 14 relative to the corresponding values at day 7. These three patients had 3 of the 4 lowest baseline IP-10 concentrations among patients experiencing a virologic rebound and the highest ratios of HCV RNA concentration at day 14 relative to that at baseline, and two showed an earlier inflection in HCV RNA concentration during danoprevir administration (data not shown).
Fig. 3.
Change in plasma IP-10 concentration and median change in HCV RNA load for patients experiencing a virologic continuous decline (A), plateau (B), or rebound (C). The change in IP-10 concentration at days 7 and 14 is shown for individual patients. Solid lines indicate the median change from baseline. The median change in HCV RNA load for the group is shown as closed gray circles connected by a solid gray line.
Neopterin concentration.
At baseline, the median neopterin plasma concentration for all patients was 7.6 nM, with a range of 4.5 to 21.3 nM (5-fold range; see Fig. S2A in the supplemental material). TN and NR patients showed median neopterin values of 7.6 and 7.7 nM, respectively. Median baseline neopterin concentrations of 8.7, 7.3, and 7.6 nM were observed in patients that experienced virologic continuous decline, plateau, and rebound, respectively (see Fig. S2B in the supplemental material). Baseline neopterin concentration was not correlated with baseline HCV RNA concentration across all patients (r2 = 0.04, P = 0.20), in TN patients (r2 = 0.02, P = 0.44), or in NRs (r2 = 0.18, P = 0.23) (see Fig. S2C in the supplemental material).
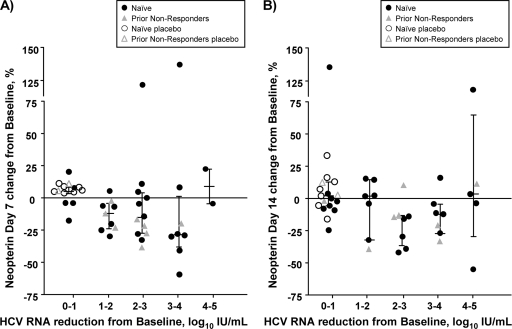
In most patients, the plasma concentration of neopterin decreased as the HCV RNA concentration decreased. However, these changes were smaller and more variable than those observed with IP-10. At day 7, patients experiencing a 1- to 2-log10-IU/ml reduction in HCV RNA concentration displayed a median reduction in neopterin concentration of 12% relative to that at baseline (Fig. 4 A). Progressively larger median decreases of 16% and 29% were observed in patients experiencing 2- to 3- and 3- to 4-log10-IU/ml reductions in HCV RNA concentration, respectively. Neopterin concentrations in the two patients experiencing the greatest reduction in HCV RNA concentration (4 to 5 log10 IU/ml) were either stable (5% reduction) or slightly higher (22% increase) than their corresponding baseline values. Similarly modest and variable reductions in median neopterin concentration were observed at day 14 (Fig. 4B).
Fig. 4.
Change in plasma neopterin concentration relative to categorical change in HCV RNA load. The percent change from baseline for individual patients at day 7 (A) or day 14 (B) is shown for five categories of change in HCV RNA. Medians and interquartile ranges are shown. Placebo-treated patients are shown as open black circles (TN patients) and open gray triangles (NRs). Danoprevir-treated patients are shown as closed black circles (TN patients) and closed gray triangles (NRs). No category of patients showed a statistically significant difference relative to those experiencing a negligible (0- to 1-log10 IU/ml) change in viral load (P < 0.05; 1-way ANOVA followed by Dunnett's test). Note the use of a discontinuous axis.
As with IP-10, the dynamics of neopterin plasma concentrations were examined separately in patients experiencing virologic continuous decline, plateau, or rebound during danoprevir administration (Fig. 5 A to C, respectively). Similar and modest reductions in median neopterin concentrations were observed in patients comprising the continuous-decline, plateau, and rebound groups at both day 7 (12.3%, 4.3%, and 25%, respectively; Fig. 5A to C) and day 14 (8.0%, 5.6%, and 7.9%, respectively; Fig. 5A to C).
Fig. 5.
Change in plasma neopterin concentration and median change in HCV RNA load for patients experiencing a virologic continuous decline (A), plateau (B), or rebound (C). The change in neopterin concentration at days 7 and 14 is shown for individual patients. Solid lines indicate the median change from baseline. The median change in HCV RNA load for the group is shown as closed gray circles connected by a solid gray line.
Correlation of IP-10 and neopterin concentrations.
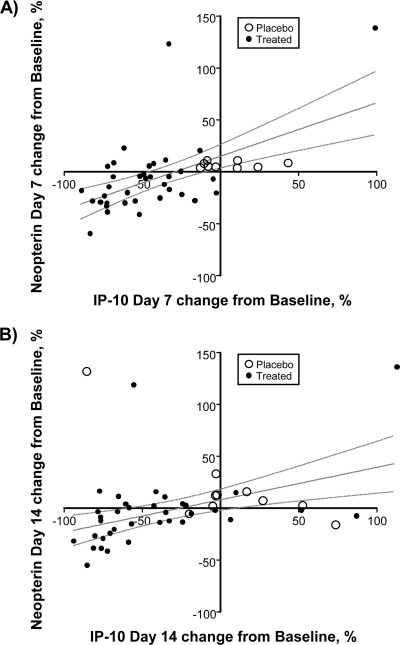
The majority of danoprevir-treated patients experienced a reduction in IP-10 plasma concentration and a lesser reduction in neopterin plasma concentration. Therefore, the data were examined to determine whether changes in IP-10 and neopterin concentrations were correlated at the patient level. A positive correlation was observed between neopterin concentration changes and IP-10 concentration changes at both day 7 (r2 = 0.35, P < 0.0001; Fig. 6 A) and day 14 (r2 = 0.21, P = 0.0017; Fig. 6B). The slopes of the associated trend lines are 0.54 and 0.31, respectively, suggesting that changes in IP-10 concentration are 2- to 3-fold greater than the corresponding change in neopterin concentration.
Fig. 6.
Correlation of changes in plasma concentrations of neopterin and IP-10. Changes in neopterin and IP-10 are shown for individual patients treated with danoprevir or placebo at day 7 (A) or day 14 (B). The solid line in each panel shows a linear fit of the data, and dashed lines show the 95% confidence limits. Changes in neopterin and IP-10 concentrations are correlated, with an r2 value of 0.35 and a P value of <0.0001 for day 7 and an r2 value of 0.21 and a P value of 0.0017 for day 14.
OAS-1 concentration.
At baseline, the median OAS-1 plasma concentration for all patients was 580 pM (see Fig. S3A in the supplemental material). Significant interpatient variability was noted, with a range 214 to 2,777 pM (13-fold). TN and NR patients showed median OAS-1 values of 580 and 561 pM, respectively. Median baseline OAS-1 concentrations of 572, 513, and 1,075 pM were observed in patients that experienced virologic continuous decline, plateau, and rebound, respectively (see Fig. S3B in the supplemental material). The range of OAS-1 values in TN and NR patients and in the continuous-decline, plateau, and rebound groups overlapped significantly, and differences between the groups were not statistically significant (see Fig. S3A and B in the supplemental material). Baseline OAS-1 concentration was not correlated with HCV RNA concentration across all patients (r2 = 0.014, P = 0.44), in TN patients (r2 = 0.0044, P = 0.70), or in NRs (r2 = 0.09, P = 0.40) (see Fig. S3C in the supplemental material).
OAS-1 plasma concentrations displayed highly variable changes relative to those at baseline at both day 7 and day 14 in patients with very little change in HCV RNA, as well as those displaying appreciable declines in HCV RNA concentration (see Fig. S4A and B in the supplemental material). No discernible differences in the dynamics of OAS-1 were observed between virologic response groups (data not shown). Furthermore, changes in OAS-1 concentration were not correlated with changes in either IP-10 or neopterin concentration (data not shown).
DISCUSSION
Danoprevir is a potent and selective inhibitor of the HCV NS3/4A serine protease that is currently in phase 2b development in combination with PEG-IFN and ribavirin and in an interferon-sparing regimen with the nucleoside polymerase inhibitor mericitabine (RG7128). In a phase 1b clinical study, 14-day danoprevir monotherapy resulted in dose-dependent antiviral effects in which the best-responding cohort of treatment-naïve patients experienced a −3.8-log10-IU/ml median reduction in HCV RNA, while a single cohort of nonresponders to previous treatment with pegylated alpha interferon-ribavirin experienced a −2.5-log10-IU/ml reduction (12).
The current study demonstrated that the antiviral effect of danoprevir monotherapy is associated with significant reductions in IP-10 plasma concentration and lesser, more variable reductions in neopterin plasma concentration. OAS-1 concentrations showed considerable variability in placebo-treated patients and those patients with little or no virologic response to danoprevir and did not appear to be modulated by changes in HCV RNA concentration.
Baseline values of plasma IP-10 varied over a 35-fold range, which is consistent with previous reports (5, 15, 22). A previous study suggested a correlation between baseline concentration of IP-10 and baseline HCV RNA using a categorical analysis in a significantly larger patient population (42). No such correlation was evidenced in this study, although this apparent discrepancy may reflect the smaller number of patients available for analysis in the current study.
In the case of interferon-based therapy, a low baseline IP-10 plasma concentration has been shown to be predictive of a strong first-phase decline in HCV RNA (3) and an improved rate of sustained virologic response (5, 8, 27, 42). Interestingly, in this study the magnitude of first-phase decline of HCV RNA during danoprevir monotherapy was directly correlated with baseline IP-10 concentration. These contrasting results may reflect the different antiviral mechanisms of interferon and a direct-acting antiviral agent or simply the small sample size in the current study.
Upon initiation of danoprevir therapy, the IP-10 plasma concentration was significantly reduced in patients that experienced a reduction in HCV RNA. These results are consistent with those of a recently published study demonstrating that patients treated for 13 days with a combination of danoprevir and the nucleoside polymerase inhibitor mericitabine showed significant reductions in IP-10 concentration (15).
In the present monotherapy study, the dynamics of IP-10 plasma concentration were further associated with changes in HCV RNA concentration in patients experiencing rebound, plateau, and continuous decline. At day 7, similar median reductions were observed for both IP-10 and HCV RNA concentrations in the rebound and continuous-decline groups. Despite a considerable divergence of changes in HCV RNA concentration between these groups at day 14, median IP-10 concentrations were similar. Notably, however, three rebound patients with an early inflection in HCV RNA concentration and/or a high HCV RNA concentration at day 14 relative to that at baseline displayed a significant rebound in IP-10 concentration at day 14. The lack of observed rebound in IP-10 concentration in the other patients experiencing a rebound in HCV RNA may suggest that increases in inflammatory responses upon viral rebound are delayed relative to increases in HCV RNA concentrations. Larger studies with extended posttreatment follow-up would be required to test this hypothesis.
In the current study, the median baseline neopterin plasma concentration of 7.6 nM is similar to concentrations previously reported for patients with chronic HCV infection (17, 19). After initiation of danoprevir treatment, the median plasma concentration of neopterin appeared to be moderately decreased, but not in a statistically significant fashion. Patients experiencing 2- to 3- and 3- to 4-log10-IU/ml reductions in HCV RNA concentrations experienced 15% and 12% median reductions in neopterin concentrations, respectively, at day 14. A previous study examined changes in neopterin concentration associated with 15-day treatment of chronic HCV patients with telaprevir (17). In that study, two identically treated patient cohorts experienced mean HCV RNA reductions of 3.8 and 4.2 log10 IU/ml. These cohorts experienced mean reductions in plasma neopterin of 14% and 30%, respectively, with the latter being statistically significant relative to that at baseline. A third cohort receiving a lower dose of telaprevir experienced a 2.5-log10-IU/ml reduction in mean HCV RNA concentration and a 13% reduction in neopterin concentration that was not statistically significant. Taken together, the results of these two studies suggest that treatment with an NS3/4A protease inhibitor promotes reduction of the plasma concentration of neopterin. However, both this study and the previous study (17) suggest that the magnitude of this effect is modest.
Changes in the plasma concentrations of IP-10 and neopterin were statistically significant but weakly correlated. The correlation between changes in IP-10 concentration and neopterin concentration is consistent with each being an inflammatory marker and their persistent elevation in patients with chronic HCV infection (5, 8, 10, 17, 19, 27, 33, 36, 52). The correlation of changes in neopterin and IP-10 suggests that these markers may have similar or overlapping roles in the assessment of HCV patients during treatment with direct-acting antiviral agents.
The median baseline OAS-1 plasma concentration of 580 pM observed in the present study is similar to that in a previous literature report (48). While the significant variability observed in placebo-treated patients over a 2-week period makes it difficult to gauge any danoprevir-related effects on OAS-1 concentration, the variability and lack of discernible correlation with antiviral response may have significant implications for the potential use of OAS-1 as a marker of response to treatment with interferon-free direct-acting antiviral regimens.
The lack of association between OAS-1 and the antiviral effect resulting from danoprevir treatment is interesting, given the hypothesis that NS3/4A can dampen viral sensing and interferon production (14, 43). With interferon-based regimens or other activators of immune responses, a significant induction of OAS-1 is observed (48). Therefore, these results suggest that direct inhibition of HCV replication in the liver does not result in a systemically evidenced interferon response.
In summary, the current study demonstrates a significant reduction in IP-10 plasma concentration in patients with an antiviral response to the NS3/4A protease inhibitor danoprevir. Reductions in IP-10 concentration during therapy correlate with reductions in neopterin concentration that are of a smaller magnitude and more variable. These results suggest that treatment with a direct-acting antiviral agent suppresses hepatic inflammation in patients with chronic HCV infection. Similar correlations were not evidenced with plasma concentrations of the interferon-stimulated gene product OAS-1.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by InterMune, Inc.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 18 April 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Adam R., et al. 2003. Evolution of liver transplantation in Europe: report of the European Liver Transplant Registry. Liver Transpl. 9:1231–1243 [DOI] [PubMed] [Google Scholar]
- 2. Anonymous 2002. NIH consensus statement on management of hepatitis C: 2002. NIH Consens. State Sci. Statements 19:1–46 [PubMed] [Google Scholar]
- 3. Askarieh G., et al. 2010. Systemic and intrahepatic interferon-gamma-inducible protein 10 kDa predicts the first-phase decline in hepatitis C virus RNA and overall viral response to therapy in chronic hepatitis. Hepatology 51:1523–1530 [DOI] [PubMed] [Google Scholar]
- 4. Bataller R., Brenner D. A. 2005. Liver fibrosis. J. Clin. Invest. 115:209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butera D., et al. 2005. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood 106:1175–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caillot F., et al. 2009. Novel serum markers of fibrosis progression for the follow-up of hepatitis C virus-infected patients. Am. J. Pathol. 175:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheung K. J., Tilleman K., Deforce D., Colle I., Van Vlierberghe H. 2009. The HCV serum proteome: a search for fibrosis protein markers. J. Viral Hepat. 16:418–429 [DOI] [PubMed] [Google Scholar]
- 8. Diago M., et al. 2006. Association of pretreatment serum interferon gamma inducible protein 10 levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1 infected patients with chronic hepatitis C. Gut 55:374–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falconer K., et al. 2010. IP-10 predicts the first phase decline of HCV RNA and overall viral response to therapy in patients coinfected with chronic hepatitis C virus infection and HIV. Scand. J. Infect. Dis. 42:896–901 [DOI] [PubMed] [Google Scholar]
- 10. Fernandez E., et al. 2000. Adenosine deaminase isoenzymes and neopterin in liver cirrhosis. J. Clin. Gastroenterol. 30:181–186 [DOI] [PubMed] [Google Scholar]
- 11. Foerster K., et al. 2010. The novel immunoregulatory molecule FGL2: a potential biomarker for severity of chronic hepatitis C virus infection. J. Hepatol. 53:608–615 [DOI] [PubMed] [Google Scholar]
- 12. Forestier N., Larrey D., Guyader D., Marcellin P., Rouzier R., Patat A., Smith P., Bradford W., Porter S., Blatt L., Seiwert S. D., Zeuzem S. 23 February 2011. Treatment of chronic hepatitis C patients with the NS3/4A protease inhibitor danoprevir (ITMN-191/RG7227) leads to robust reductions in viral RNA: a phase 1b multiple ascending dose study. J. Hepatol. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 13. Fried M. W., et al. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975–982 [DOI] [PubMed] [Google Scholar]
- 14. Gale M., Jr., Foy E. M. 2005. Evasion of intracellular host defense by hepatitis C virus. Nature 436:939–945 [DOI] [PubMed] [Google Scholar]
- 15. Gane E. J., et al. 2010. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet 376:1467–1475 [DOI] [PubMed] [Google Scholar]
- 16. Ge D., et al. 2009. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461:399–401 [DOI] [PubMed] [Google Scholar]
- 17. Gelderblom H. C., et al. 2008. Inflammatory markers neopterin and alanine aminotransferase in HCV patients treated with HCV NS3.4A protease inhibitor telaprevir (VX-950) and/or peginterferon alfa-2a. Scand. J. Gastroenterol. 43:1122–1127 [DOI] [PubMed] [Google Scholar]
- 18. Grebely J., et al. 2010. Potential role for interleukin-28B genotype in treatment decision-making in recent hepatitis C virus infection. Hepatology 52:1216–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grungreiff K., Reinhold D., Ansorge S. 1999. Serum concentrations of sIL-2R, IL-6, TGF-beta1, neopterin, and zinc in chronic hepatitis C patients treated with interferon-alpha. Cytokine 11:1076–1080 [DOI] [PubMed] [Google Scholar]
- 20. Hamerlinck F. F. 1999. Neopterin: a review. Exp. Dermatol. 8:167–176 [DOI] [PubMed] [Google Scholar]
- 21. Harvey C. E., et al. 2003. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J. Leukoc. Biol. 74:360–369 [DOI] [PubMed] [Google Scholar]
- 22. Helbig K. J., et al. 2009. Differential expression of the CXCR3 ligands in chronic hepatitis C virus (HCV) infection and their modulation by HCV in vitro. J. Virol. 83:836–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang W., et al. 2010. Plasma osteopontin concentration correlates with the severity of hepatic fibrosis and inflammation in HCV-infected subjects. Clin. Chim. Acta 411:675–678 [DOI] [PubMed] [Google Scholar]
- 24. Huber C., et al. 1984. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J. Exp. Med. 160:310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanmura S., et al. 2009. The complement component C3a fragment is a potential biomarker for hepatitis C virus-related hepatocellular carcinoma. J. Gastroenterol. 45:459–467 [DOI] [PubMed] [Google Scholar]
- 26. Knapp S., et al. 2003. Polymorphisms in interferon-induced genes and the outcome of hepatitis C virus infection: roles of MxA, OAS-1 and PKR. Genes Immun. 4:411–419 [DOI] [PubMed] [Google Scholar]
- 27. Lagging M., et al. 2006. IP-10 predicts viral response and therapeutic outcome in difficult-to-treat patients with HCV genotype 1 infection. Hepatology 44:1617–1625 [DOI] [PubMed] [Google Scholar]
- 28. Larrubia J. R., Benito-Martínez S., Calvino M., Sanz-de-Villalobos E., Parra-Cid T. 2008. Role of chemokines and their receptors in viral persistence and liver damage during chronic hepatitis C virus infection. World J. Gastroenterol. 14::7149–7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larrubia J. R., et al. 2007. The role of CCR5/CXCR3 expressing CD8+ cells in liver damage and viral control during persistent hepatitis C virus infection. J. Hepatol. 47:632–641 [DOI] [PubMed] [Google Scholar]
- 30. Li C. Z., et al. 2009. Polymorphism of OAS-1 determines liver fibrosis progression in hepatitis C by reduced ability to inhibit viral replication. Liver Int. 29:1413–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moura A. S., Carmo R. A., Teixeira A. L., Otávio da Costa Roch M. 2009. Soluble inflammatory markers as predictors of hepatocellular damage and therapeutic response in chronic hepatitis C. Braz. J. Infect. Dis. 13:375–382 [DOI] [PubMed] [Google Scholar]
- 32. Mullan P. B., et al. 2005. The 2,5 oligoadenylate synthetase/RNaseL pathway is a novel effector of BRCA1- and interferon-gamma-mediated apoptosis. Oncogene 24:5492–5501 [DOI] [PubMed] [Google Scholar]
- 33. Narumi S., et al. 1997. Expression of IFN-inducible protein-10 in chronic hepatitis. J. Immunol. 158:5536–5544 [PubMed] [Google Scholar]
- 34. Neville L. F., Mathiak G., Bagasra O. 1997. The immunobiology of interferon-gamma inducible protein 10 kD novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 8:207–219 [DOI] [PubMed] [Google Scholar]
- 35. Ngo Y., et al. 2006. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin. Chem. 52:1887–1896 [DOI] [PubMed] [Google Scholar]
- 36. Nubling C. M., Chudy M., Volkers P., Lower J. 2006. Neopterin levels during the early phase of human immunodeficiency virus, hepatitis C virus, or hepatitis B virus infection. Transfusion 46:1886–1891 [DOI] [PubMed] [Google Scholar]
- 37. Patzwahl R., Meier V., Ramadori G., Mihm S. 2001. Enhanced expression of interferon-regulated genes in the liver of patients with chronic hepatitis C virus infection: detection by suppression-subtractive hybridization. J. Virol. 75:1332–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pawlotsky J. M., et al. 1995. Activity of the interferon-induced 2′,5′-oligoadenylate synthetase in patients with chronic hepatitis C. J. Interferon Cytokine Res. 15:857–862 [DOI] [PubMed] [Google Scholar]
- 39. Pineda J. A., et al. 2010. Prediction of response to pegylated interferon plus ribavirin by IL28B gene variation in patients coinfected with HIV and hepatitis C virus. Clin. Infect. Dis. 51:788–795 [DOI] [PubMed] [Google Scholar]
- 40. Rehermann B. 2009. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J. Clin. Invest. 119:1745–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reiberger T., et al. 2008. IP-10 correlates with hepatitis C viral load, hepatic inflammation and fibrosis and predicts hepatitis C virus relapse or non-response in HIV-HCV coinfection. Antivir. Ther. 13:969–976 [PubMed] [Google Scholar]
- 42. Romero A. I., et al. 2006. Interferon (IFN)-gamma-inducible protein-10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J. Infect. Dis. 194:895–903 [DOI] [PubMed] [Google Scholar]
- 43. Saito T., Gale M., Jr 2008. Regulation of innate immunity against hepatitis C virus infection. Hepatol. Res. 38:115–122 [DOI] [PubMed] [Google Scholar]
- 44. Sarrazin C., et al. 2009. Kinetic analysis of viral rebound and drug-resistant viral variant dynamics in patients treated with ITMN-191 (R7227) monotherapy suggest a high barrier to viral escape. Hepatology 50:953A–954A [Google Scholar]
- 45. Seeff L. B., Hoofnagle J. H. 2002. National Institutes of Health Consensus Development Conference: management of hepatitis C: 2002. Hepatology 36:s1–s2 [DOI] [PubMed] [Google Scholar]
- 46. Seiwert S. D., et al. 2008. Preclinical characteristics of the hepatitis C virus NS3/4A protease inhibitor ITMN-191 (R7227). Antimicrob. Agents Chemother. 52:4432–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shepard C. W., Finelli L., Alter M. J. 2005. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 5:558–567 [DOI] [PubMed] [Google Scholar]
- 48. Solinas A., et al. 1993. Changes of serum 2′,5′-oligoadenylate synthetase activity during interferon treatment of chronic hepatitis C. Liver 13:253–258 [DOI] [PubMed] [Google Scholar]
- 49. Taub D. D., et al. 1993. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J. Exp. Med. 177:1809–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Terrault N., et al. 2010. Phase II randomised, partially-blind, parallel group study of oral danoprevir (RG7227) with PEGIFNα-2A (PEGASYS®) plus ribavirin in treatment-naive genotype 1 patients with CHC: results of a planned week 12 interim analysis of the ATLAS Study. Hepatology 52:335A–336A [Google Scholar]
- 51. Welzel T. M., et al. 2009. Variants in interferon-α pathway genes and response to pegylated-interferon-α2a plus ribavirin for treatment of chronic HCV infection in the HALT-C Trial. Hepatology 49:1847–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilmer A., et al. 1995. Serum neopterin concentrations in chronic liver disease. Gut 37:108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu X., et al. 2009. A pharmacogenetic study of polymorphisms in interferon pathway genes and response to interferon-alpha treatment in chronic hepatitis B patients. Antiviral Res. 83:252–256 [DOI] [PubMed] [Google Scholar]
- 54. Zeremski M., et al. 2009. Peripheral CXCR3-associated chemokines as biomarkers of fibrosis in chronic hepatitis C virus infection. J. Infect. Dis. 200:1774–1780 [DOI] [PubMed] [Google Scholar]
- 55. Zeremski M., et al. 2008. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology 48:1440–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zeremski M., Petrovic L. M., Talal A. H. 2007. The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. J. Viral Hepat. 14:675–687 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.