Abstract
We developed a novel model of onychomycosis in which we observed fungi in the deep layer of the nail, and we used the model to evaluate the efficacy of two topical antifungal drugs. To establish an experimental, in vivo model of onychomycosis, we applied Trichophyton mentagrophytes TIMM2789 to the nails of the hind limbs of rabbits that underwent steroid treatment. The nails were taken from the rabbits' feet at 0, 2, and 6 weeks after a 2-week infection. The localization of the fungi was evaluated histopathologically. Some fungi were seen to penetrate to the nail bed, and the infection rate in the sample at 0, 2, and 6 weeks after infection was 57, 87, and 93%, respectively. In addition, fungi proliferated and moved proximally into the nail plate in a manner that depended on the duration of infection. Second, using this model we evaluated antifungal efficacy both by the culture recovery method and histopathological examination. Two topical antifungal drugs, 8% ciclopirox nail lacquer and 5% amorolfine nail lacquer, were applied to the nail for 4 weeks in each group. On histopathological examination, two antifungal treatment groups showed no significant difference against the nontreated control group. However, there were a significantly low fungus-positive rate and intensity of the recovery of fungi on culture between antifungal treatment and nontreated control groups. We therefore suggest that we have established an in vivo model of onychomycosis that is useful for the evaluation of the efficacy of antifungal agents.
INTRODUCTION
Onychomycosis is an intractable superficial mycosis, and the oral administration of antifungal agents is the main modality for clinical treatment (9). However, some of these oral antifungal drugs have well-known drug interactions with other medications, and these interactions limit their usage in older people and those living with diabetes or human immunodeficiency virus (3). These patients eagerly await topical antifungal agents that are both effective and useful.
Tatsumi et al. (21) reported the evaluation of some antifungal agents using an in vivo experimental model of onychomycosis, but detailed histological examination was limited, and they did not describe whether fungal infection occurred near the nail bed, as occurs in the clinical setting. Drug efficacy tests that used this model were useful in the evaluation of orally administered drugs but not for topical agents. Agents that are active following oral administration reach the nail bed via the circulation and then diffuse toward the dorsal surface of the nail. Consequently, if the fungal infection that occurs in this animal model is only superficial, where the lowest concentration of active agent is thought to be that following oral administration, drug efficacy can be evaluated adequately. To evaluate topical drugs using this model, we need to confirm its efficacy in the deeper layers of the nail.
Although there are reports that describe histological findings in human onychomycosis and that confirm the value of histopathological examination in making the diagnosis (4, 11, 17, 19), the route of infection and how the fungi behave in the nail plate have not been determined in detail.
The rate of morbidity in onychomycosis may be affected by age, smoking, peripheral arterial disease, diabetes, and immunodeficiency (6, 15, 23). In particular, a multicenter survey (8) reports that the administration of immunosuppressive agents to people with diabetes may be an important factor that predisposes patients to onychomycosis.
In this article, we attempt to establish an animal model of onychomycosis in immunosuppressed rabbits using Trichophyton mentagrophytes, a well-recognized and widely identified pathogen in rabbits (1, 10, 22, 25). We used histopathological examination and the culture recovery method to evaluate the efficacy of 8% ciclopirox nail lacquer (Penlac; Dermik Laboratories, Sanofi-Aventis, Bridgewater, NJ) and 5% amorolfine nail lacquer (Loceryl; Galderma, Lausanne, Switzerland), which are referred to as topical onychomycosis treatments in our model.
MATERIALS AND METHODS
In this article, the terminology we use in describing the histology of the nail tissue of the rabbit is identical to that used for humans.
Animals.
Male Japanese white rabbits, aged 14 weeks, were purchased from Kitayama Labes Co., Ltd., and used in this study. The experiment to establish the animal onychomycosis model was performed in three groups with five rabbits in each group, while the experiment that determined therapeutic efficacy was performed in three groups with four rabbits in each group. The nails examined were on the first to third toes of the right and left hind paws; that is, six nails were used per animal. All experimental procedures were evaluated and approved in accordance with the Institutional Animal Care and Use Committee (IACUC) of Pola Pharma Inc.
Test organism.
T. mentagrophytes TIMM2789, isolated from a guinea pig, was purchased from Teikyo University Institute of Medical Mycology, Tokyo, Japan.
Preparation of inocula.
Freeze-dried T. mentagrophytes was suspended in 1 ml of saline containing 0.05% Tween 80. A volume of 0.05 ml of this suspension was seeded onto fluid Sabouraud dextrose agar, and T. mentagrophytes was cultured at 28°C for 2 weeks to prepare microconidia. After the fungi were subcultured, microconidia of T. mentagrophytes were taken from the fungi in saline, and the suspension of microconidia was adjusted to give a concentration of 108 conidia/ml by counting with a hemocytometer.
Production of onychomycosis.
Four mg/kg of body weight of methylprednisolone acetate was injected intramuscularly into the hind limb of each rabbit once a week for 4 weeks until the end of the infection period. In some cases, the dosage of steroid was decreased depending on the condition of the animal. Two weeks after starting steroid treatment, 0.2 ml of microconidia suspension was dripped onto the nail at a site between the lunula and the proximal nail fold. A gauze patch then was used to wrap together the nail plates of the first to third toes of the hind paw. The treated toe nails were covered with a finger cot (that contained the first to third toes), and 0.5 ml of sterile water was injected into the finger cot to produce a culture environment around the nail that was suitable for fungal growth. This condition was maintained for a period of infection of 2 weeks without any other intervention. The finger cot and the gauze patch were removed after 2 weeks of exposure, and this condition was maintained for 0, 2, or 6 weeks without finger cot and gauze patch; this was termed the postinfection period. After each postinfection period was completed, the animals were sacrificed and the nails were taken from the treated paw for histopathological and microbiological examination.
Histopathological examination of nail tissue.
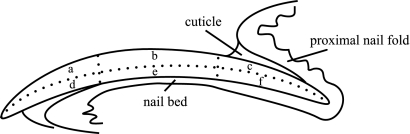
The nails sampled for histopathological examination were fixed in 20% (vol/vol) buffered neutral formalin solution for 1 week and decalcified in 5% (vol/vol) formalin solution containing 5% (vol/vol) buffered formic acid for 1 week. They were neutralized in 5% (vol/wt) sodium sulfate aqueous solution for 1 week and embedded in paraffin. The nails were sectioned using a microtome with the paraffin tape transfer system (Instrumedics, Hackensack, NJ) to support the cohesion of whole-nail elements. Thin paraffin sections of the nails were observed by light microscopy after being stained with the periodic acid-Schiff stain. The following semiquantitative scoring system was used to describe the intensity of T. mentagrophytes infection in the nail plate: grade 0, no fungus; grade 1, fungi present but not forming a cluster; grade 2, a few clusters of fungi present; and grade 3, numerous clusters of fungi present. The infection rate was calculated as follows. The number of nails in which the grade was above 1 was divided by the total number of nails evaluated in each group. The histological localization of fungi in the nail plate was evaluated based on the intensity and the infection rate in each of six regions, that is, following division in two in a lengthwise direction from the dorsum of the nail plate to the nail bed and in three across from the proximal to distal end of the nail plate (Fig. 1).
Fig. 1.
Diagram of histopathology of rabbit nail. The histological examination was carried out according to the six regions separated: a, dorsal and distal; b, dorsal and medium; c, dorsal and proximal; d, ventral and distal; e, ventral and medium; and f, ventral and proximal side of nail plate.
Drugs and treatment.
In this study, 8% ciclopirox nail lacquer and 5% amorolfine nail lacquer were purchased through an import company and used as test items. Each nail was cleaned with water-soaked cotton just before the application of the drug, and then a volume of 3.6 μl of each drug was applied topically to the nails. The 8% ciclopirox nail lacquer was applied once a day, and 5% amorolfine nail lacquer was applied twice a week according to the drug package insert. Applications of these drugs were carried out from the day following the end of the period of infection for 28 days. The animals in the untreated control group underwent the process of infection and the removal of the material used for this process, but they were not exposed to the test agents. In cases that underwent the drug efficacy experiment, the nails sampled from the paw were cut in two lengthwise, and one piece was used for histopathological evaluation while the second was used for evaluation using culture recovery.
Evaluation of therapeutic efficacy using the culture recovery method.
The infected nail intended for evaluation using culture recovery was cut into 10 pieces in cross-section. Each nail piece was implanted onto a plate of Sabouraud dextrose agar (Difco Laboratories, Detroit, MI) containing, in 1 liter, 0.5 g of cycloheximide, 100 mg of chloramphenicol, and 50 mg of sisomicin. All plates with implanted nails were incubated for 2 weeks at 28°C. A nail piece that had confirmed fungal growth was assessed as culture positive, and a nail with more than one culture-positive piece was considered fungus positive. The extent of fungal burden was assessed with scores ranging from +10 to 0, based on the number of culture-positive nail pieces out of all 10 pieces examined.
Statistical analysis.
The total infection rate by histopathological examination and the frequency of fungus-positive nails by the culture recovery method in each group was analyzed using Fisher's exact test. The intensities of the fungal burden of the infected nails in each group were analyzed by Student's t test. P values of less than 0.05 were regarded as significant.
RESULTS
Establishment of an onychomycosis model using rabbits.
After a 2-week infection period under immunosuppression, the postinfection period was set to 0, 2, and 6 weeks in each group to determine a suitable postinfection period. Some of the infected nails became cloudy on gross appearance, which is similar to the findings with human onychomycosis (Fig. 2). With a longer postinfection period, these findings were fully confirmed. A correlation between the gross appearance and histopathological changes, however, was not confirmed as clearly. On histopathological examination, hyphae of T. mentagrophytes were seen to penetrate the nail plate, and some invading fungi reached the nail bed (Fig. 3). Most hyphae have a septum, and some of them were thin and were crushed within the nail layer. Chains of spores also were seen near the surface of the nail (Fig. 3). The total infection rate at 0, 2, and 6 weeks into the postinfection period was 56.7 (17/30), 86.7 (26/30), and 93.3% (28/30), respectively, and the regions that showed the highest infection rate in the six divided portions at each postinfection period were the proximal/dorsal side (46.7%), the distal/ventral side (62.1%), and the distal/ventral side (90.0%), respectively (Table 1 ). The regions that showed the highest infection intensity also were the proximal/dorsal side (0.73), distal/ventral side (1.45), and distal/ventral side (2.53) in each group. Additionally, the presence of subungual abscess with associated necrosis of the epithelium of the nail bed or matrix was confirmed near the fungi in the nail plate (Fig. 3). For subungual abscesses, changes in the appearance rate with time were similar to those for the infection rate. Therefore, the total appearance rates of subungual abscesses in the groups at 0, 2, and 6 weeks postinfection were 33.3 (10/30), 43.3 (13/30), and 93.3% (28/30), respectively (Table 1).
Fig. 2.
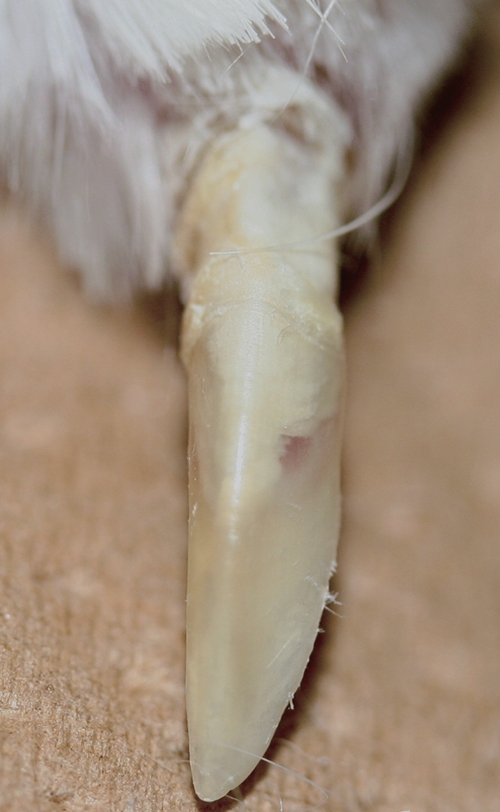
Gross appearance of infected nails at 6 weeks postinfection in an experiment to establish a model of onychomycosis. A cloudy appearance like that of human onychomycosis was observed in some infected nails.
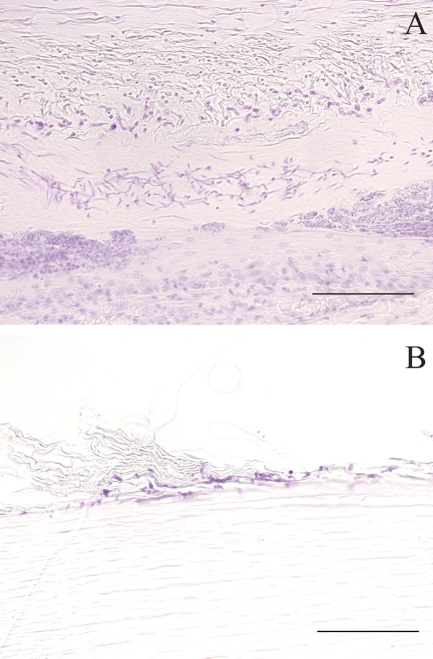
Fig. 3.
Histological findings in infected nails at 6 weeks postinfection (A) and 2 weeks postinfection (B). Many fungi and a cluster of necrotic tissue in the nail deep layers were observed (A), and some of the infected nails showed chains of spores near the surface of the nail (B). Bar = 100 μm.
Table 1.
Localization of fungi, total infection rate, and frequency of subungual abscess
| Postinfection period (wk) and locatione | Infection ratea (%) |
Total infection rateb (%) | Appearance rate of subungual abscessesc (%) |
Total appearance rate of subungual abscessesd (%) | ||
|---|---|---|---|---|---|---|
| Dorsal side | Ventral side | Dorsal side | Ventral side | |||
| 0 | ||||||
| D | 0.0 (0.00)f | 13.3 (0.23) | 56.7 | 0.0 | 0.0 | 33.3 |
| M | 6.7 (0.07) | 23.3 (0.43) | 0.0 | 23.3 | ||
| P | 46.7 (0.73) | 36.7 (0.67) | 6.7 | 16.7 | ||
| 2 | ||||||
| D | 0.0 (0.00) | 62.1 (1.45) | 86.7 | 0.0 | 13.8 | 43.3 |
| M | 17.2 (0.38) | 37.9 (1.03) | 0.0 | 37.9 | ||
| P | 43.3 (0.5) | 30 (0.47) | 3.3 | 13.3 | ||
| 6 | ||||||
| D | 40 (0.97) | 90 (2.53) | 93.3 | 33.3 | 90 | 93.3 |
| M | 50 (1.2) | 46.7 (1.13) | 33.3 | 46.7 | ||
| P | 20 (0.4) | 13.3 (0.37) | 6.7 | 6.7 | ||
Infection rate was determined with the formula (number of histologically fungus-positive nails in each region/number of nails tested in each region) × 100.
Total infection rate was determined with the formula (number of histologically fungus-positive nails/number of nails tested) × 100.
Localization of appearance rate was determined with the formula (number of nails with subungual abscesses in each region/number of nails tested in each region) × 100.
Total appearance rate was determined with the formula (number of nails with subungual abscesses/number of nails tested) × 100.
D, distal; M, middle; P, proximal.
Numbers in parentheses are averages of infection intensity grades in each region.
Histopathological evaluation of the drug efficacy in our onychomycosis model.
Once-daily topical treatment with 8% ciclopirox nail lacquer or twice-a-week topical treatment with 5% amorolfine nail lacquer was applied to infected nails. Total infection rates in the control, 8% ciclopirox nail lacquer, and 5% amorolfine nail lacquer groups were 66.7 (16/24), 47.6 (10/21), and 43.5% (10/23), respectively. The regions that showed the highest infection rate in the six sections of each group were the distal/ventral side (65.2%), distal/ventral side (33.3%), and medium/ventral side (30.4%), respectively (Table 2). The region where the intensity of infection was greatest was the distal/ventral side (1.83, 0.90, and 0.67); it was the same in all groups. Additionally, the total appearance rates for subungual abscesses in the control, 8% ciclopirox nail lacquer, and 5% amorolfine nail lacquer groups were 58.3 (14/24), 38.1 (8/21), and 39.1% (9/23), respectively (Table 2). In statistical analysis, however, no significant difference was found in the total infection rate between drug treatment groups and the nontreated control group.
Table 2.
Localization of fungi, total infection rate, and frequency of subungual abscesses in experimental evaluation of drug efficacy
| Treatment and locatione | Infection ratea (%) |
Total infection rateb (%) | Appearance rate of subungual abscessesc (%) |
Total appearance rate of subungual abscessesd (%) | ||
|---|---|---|---|---|---|---|
| Dorsal side | Ventral side | Dorsal side | Ventral side | |||
| None | ||||||
| D | 0.0 (0.00)f | 65.2 (1.83) | 66.7 | 0.0 | 47.8 | 58.3 |
| M | 26.1 (0.54) | 34.8 (0.86) | 21.7 | 43.5 | ||
| P | 12.5 (0.13) | 4.2 (0.13) | 4.2 | 4.2 | ||
| 8% Ciclopirox nail lacquer | ||||||
| D | 4.8 (0.13) | 33.3 (0.9) | 47.6 | 4.8 | 33.3 | 38.1 |
| M | 19 (0.29) | 19 (0.35) | 9.5 | 14.3 | ||
| P | 14.3 (0.13) | 9.5 (0.08) | 4.8 | 0.0 | ||
| 5% Amorolfine nail lacquer | ||||||
| D | 4.3 (0.13) | 26.1 (0.67) | 43.5 | 4.3 | 26.1 | 39.1 |
| M | 8.7 (0.17) | 30.4 (0.63) | 4.3 | 30.4 | ||
| P | 13.6 (0.18) | 4.5 (0.13) | 0.0 | 0.0 | ||
Infection rate was determined with the formula (number of histologically fungus-positive nails in each region/number of nails tested in each region) × 100.
Total infection rate was determined with the formula (number of histologically fungus-positive nails/number of nails tested) × 100.
Localization of appearance rate was determined with the formula (number of nails with subungual abscesses in each region/number of nails tested in each region) × 100.
Total appearance rate was determined with the formula (number of nails with subungual abscesses/number of nails tested) × 100.
D, distal; M, middle; P, proximal.
Numbers in parentheses are averages of infection intensity grades in each region.
Microbiological evaluation of drug efficacy in our onychomycosis model.
The culture recovery method was used for the microbiological evaluation of the antifungal effect of the two reference drugs in the nail. Table 3 shows the recovery rate and fungal burden of infected nails. The recovery rate in the untreated group was 75% (18/24). Conversely, those of the 8% ciclopirox nail lacquer and 5% amorolfine nail lacquer groups were 66.7 (16/24) and 20.8% (5/24), respectively. Furthermore, a statistically significantly lower rate was found in the 5% amorolfine nail lacquer group for comparing the infection rate to that in both the control and 8% ciclopirox nail lacquer groups and, likewise, for comparing the infection intensity to that of the control group.
Table 3.
Culture recovery: evaluation of efficacy of two topical antifungal drugs
| Treatment | Duration of treatment (days) | No. of fungus-positive nails/total no. of nails (%) | Avg fungal burden of infected nails |
|---|---|---|---|
| None | 28 | 18/24 (75.0) | +4.4 |
| 8% Ciclopirox nail lacquer | 28 | 16/24 (66.7) | +2.8 |
| 5% Amorolfine nail lacquer | 28 | 5/24a (20.8) | +0.4b |
P < 0.01 versus the untreated control and 8% ciclopirox nail lacquer-treated groups.
P < 0.01 versus the untreated control group.
DISCUSSION
The first report of an animal model for onychomycosis was described by Tatsumi et al. in 2002, with the use of guinea pigs. These authors also used this model to evaluate the efficacy of a topical antifungal agent, but the article did not describe the pathophysiological findings in detail, omitting how the gross appearance changed as well as the histopathological findings on the examination of the infected nails (21). Thus, it was not clear whether the drug efficacy found using the animal model reflected the drug's therapeutic effect against onychomycosis in clinical practice.
In the clinical therapy of onychomycosis, a poor outcome of the use of topical antifungals has been mentioned, and most research attributes this to their poor penetration onto the nail plate (20, 24, 26). Some reports suggest that the nail has properties like those of a hydrophilic gel membrane (13, 16), which prevents the penetration of the nail plate by chemicals and drugs (5). In addition, the histopathological examination of nail biopsy specimens revealed the presence of fungi near the distal and proximal nail bed in the distal subungual type (DSO) (11). In animal models in which topical drug efficacy for onychomycosis is evaluated, it is preferable that the causative organism is able to invade the deeper layers of the nail to confirm drug efficacy in this region.
In this study, our experimental animal model succeeded in encouraging T. mentagrophytes to invade the deeper layers of the nail plate, and this was clearly confirmed by our histopathological examination. Furthermore, our model allowed the assessment of growth in the nail plate and changes in the localization of the fungi. This is the first report of fungal behavior in the nail plate in an experimental animal model of onychomycosis.
Haneke (11) has investigated the localization of infected fungi on the longitudinal section of the plate and adjacent soft tissue from patients with subungual onychomycosis of both proximal and distal subtypes, and he reported that the proximal subtypes showed a high density of fungal cells from the eponychium to the proximal nail bed, and distal subtypes showed that from the nail matrix to the distal nail bed. In our study, the regions seriously involved in fungal infection were the proximal nail plate in the group immediately postinfection (0 weeks) and the distal nail plate at 2 and 6 weeks. This suggests that the findings in our model are similar to the clinical diagnoses of proximal subungual type (PSO) and DSO, and that a transformation from PSO to DSO depends on the interval that follows infection. However, a few of chains of spores also were identified near the nail surface. This finding has been reported as superficial onychomycosis in humans (11). Our model therefore also could partially recreate this type of superficial onychomycosis.
Of relevance is that the proximal nail fold was thought to be the point of invasion of dermatophytes into the nail in PSO (27). Another report of a human experiment (2) showed a similar finding, in that the lunula was the most susceptible area. In this study, the proximal/dorsal side was the most common area of involvement of fungal infection, revealing the highest infection rate immediately after the end of the infection period, a finding that supports the suggestion described above.
In our model, fungi within the nail plate did not move in the direction opposite of nail growth. The period of drug treatment therefore may be limited, because in this model there is a possibility of complete cure over a longer experimental period. In this model, however, it was confirmed that the causative organism was present for at least 6 weeks after the infection period. We therefore confirmed that our model could be used to conduct a drug efficacy test for onychomycosis within this interval.
As noted above, in this drug efficacy experiment we used a drug treatment period of 4 weeks. Some of the infected nails became cloudy on gross appearance; however, the gross appearance of the infected nails and the shape of fungal cells did not clearly differentiate between groups with and without the treatment. The total infection rate clarified by histopathological examination showed no statistically significant difference in drug-treated groups compared to that of the nontreated control group. However, a statistically significant reduction was observed by the recovery culture method. A possible reason for the difference in the results of histopathological examination and the culture recovery method is the limitations of classical histological staining and semiquantitative histopathological scoring. In particular, carbohydrates originating from fungal walls were intensely stained by the periodic acid-Schiff stain even with the reduced viability of the fungi (12). Therefore, it may be that the outcome of histopathological examination could not reflect the real drug efficacy.
On the other hand, the culture-positive rate in controls, 8% ciclopirox nail lacquer, and 5% amorolfine nail lacquer was 75.0, 66.7, and 20.8%, respectively. It has been reported that the clinical cure rate for 5% amorolfine nail lacquer was 38 to 52% (14, 18), and that of 8% ciclopirox nail lacquer was 8% (7). When each rate of culture positivity in the drug-treated groups was subtracted from that in the nontreated group, the differences were 54.2% for 5% amorolfine nail lacquer and 8.3% for 8% ciclopirox nail lacquer. This figure was similar in the clinical reports mentioned above. Moreover, the infection rate and frequency of subungual abscesses that were calculated based on the histological findings in drug-treated groups were low compared to those in the control group, and these frequencies were similar in the 8% ciclopirox nail lacquer or 5% amorolfine nail lacquer treatment groups. The efficacy of these drugs thus was detected using this animal model. In addition, a difference in the efficacy of 8% ciclopirox nail lacquer and 5% amorolfine nail lacquer was confirmed by the culture recovery methods.
In general, subungual abscesses are thought to be rare in humans with onychomycosis. Our procedure induced subungual abscesses, which resulted mostly from an induced immunocompromised condition. Whereas the model usually is complicated with subungual abscess formation, the pathophysiology of the onychomycosis of subungual subtypes in humans infected in the deep layer of the nail is faithfully reproduced, which includes the site of initial proliferation (cuticle) and the manner of supply, movement, positioning, and proliferation of fungi in the nail plate. Therefore, the fungal dynamics that were confirmed in our model were thought to be suitable for the evaluation of an antifungal agent, especially topical ones.
Moreover, the evaluation of topical drug efficacy by the culture recovery method confirmed results similar to those encountered in a clinical setting. We therefore have established an animal model of onychomycosis and confirmed that our model can be useful in evaluating the efficacy of antifungals.
ACKNOWLEDGMENTS
This work was supported by Health Science Research Grants for Research on Emerging and Reemerging Infectious Diseases (H16-Shinko-6 and H19-Shinko-8) and Measures for Intractable Diseases (H20 nannchi ippann 35) from the Ministry of Health, Labor and Welfare of Japan; a grant of the strategic basis on research grounds for nongovernmental schools in 2008 from the Ministry of Education, Culture, Sports, Science and Technology-Japan to K.S.; and the grant of the strategic basis on research grounds for nongovernmental schools at Heisei 20th from the Ministry of Education, Culture, Sports, Science and Technology-Japan. K.S. received research grants from Pfizer Inc., Janssen Pharmaceutical K.K., and Dainippon Sumitomo Pharma Co.
We declare that we have no competing interests.
Footnotes
Published ahead of print on 9 May 2011.
REFERENCES
- 1. Cabañes F. J., Abarca M. L., Bragulat M. R. 1997. Dermatophytes isolated from domestic animals in Barcelona, Spain. Mycopathologia 137:107–113 [DOI] [PubMed] [Google Scholar]
- 2. Casanovas M., Francino J., Vilanova X. 1956. Onychomycosis; an experimental study. J. Investig. Dermatol. 27:77–101 [PubMed] [Google Scholar]
- 3. Elewski B., Tavakkol A. 2005. Safety and tolerability of oral antifungal agents in the treatment of fungal nail disease: a proven reality. Ther. Clin. Risk Manag. 1:299–306 [PMC free article] [PubMed] [Google Scholar]
- 4. Gianni C., et al. 2001. Usefulness of histological examination for the diagnosis of onychomycosis. Dermatology 202:283–288 [DOI] [PubMed] [Google Scholar]
- 5. Gniadecka M., Faurskov Nielsen O., Christensen D. H., Wulf H. C. 1998. Structure of water, proteins, and lipids in intact human skin, hair, and nail. J. Investig. Dermatol. 110:393–398 [DOI] [PubMed] [Google Scholar]
- 6. Gupta A. K., et al. 2000. The epidemiology of onychomycosis: possible role of smoking and peripheral arterial disease. J. Eur. Acad. Dermatol. Venereol. 14:466–469 [DOI] [PubMed] [Google Scholar]
- 7. Gupta A. K., Joseph W. S. 2000. Ciclopirox 8% nail lacquer in the treatment of onychomycosis of the toenails in the United States. J. Am. Podiatr. Med. Assoc. 90:495–501 [DOI] [PubMed] [Google Scholar]
- 8. Gupta A. K., et al. 1998. Prevalence and epidemiology of toenail onychomycosis in diabetic subjects: a multicentre survey. Br. J. Dermatol. 139:665–671 [DOI] [PubMed] [Google Scholar]
- 9. Gupta A. K., Uro M., Cooper E. A. 2010. Onychomycosis therapy: past, present, future. J. Drugs Dermatol. 9:1109–1113 [PubMed] [Google Scholar]
- 10. Hagen K. W., Gorham J. R. 1972. Dermatomycoses in fur animals: chinchilla, ferret, mink and rabbit. Vet. Med. Small Anim. Clin. 67:43–48 [PubMed] [Google Scholar]
- 11. Haneke E. 1985. Nail biopsies in onychomycosis. Mykosen 28:473–480 [DOI] [PubMed] [Google Scholar]
- 12. Kligman A. M., Mescon H. 1950. The periodic acid-Schiff stain for the demonstration of fungi in animal tissue. J. Bacteriol. 60:415–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kobayashi Y., Miyamoto M., Sugibayashi K., Morimoto Y. 1999. Drug permeation through the three layers of the human nail plate. J. Pharm. Pharmacol. 51:271–278 [DOI] [PubMed] [Google Scholar]
- 14. Lauharanta J. 1992. Comparative efficacy and safety of amorolfine nail lacquer 2% versus 5% once weekly. Clin. Exp. Dermatol. 17(Suppl. 1):41–43 [DOI] [PubMed] [Google Scholar]
- 15. Levy L. A. 1997. Epidemiology of onychomycosis in special-risk populations. J. Am. Podiatr. Med. Assoc. 87:546–550 [DOI] [PubMed] [Google Scholar]
- 16. Mertin D., Lippold B. C. 1997. In-vitro permeability of the human nail and of a keratin membrane from bovine hooves: influence of the partition coefficient octanol/water and the water solubility of drugs on their permeability and maximum flux. J. Pharm. Pharmacol. 49:30–34 [DOI] [PubMed] [Google Scholar]
- 17. Moreno-Coutiño G., Toussaint-Caire S., Arenas R. 2010. Clinical, mycological and histological aspects of white onychomycosis. Mycoses 53:144–147 [DOI] [PubMed] [Google Scholar]
- 18. Reinel D., Clarke C. 1992. Comparative efficacy and safety of amorolfine nail lacquer 5% in onychomycosis, once-weekly versus twice-weekly. Clin. Exp. Dermatol. 17(Suppl. 1):44–49 [DOI] [PubMed] [Google Scholar]
- 19. Reisberger E. M., Abels C., Landthaler M., Szeimies R. M. 2003. Histopathological diagnosis of onychomycosis by periodic acid-Schiff-stained nail clippings. Br. J. Dermatol. 148:749–754 [DOI] [PubMed] [Google Scholar]
- 20. Scher R. K., Baran R. 2003. Onychomycosis in clinical practice: factors contributing to recurrence. Br. J. Dermatol. 149(Suppl. 65):5–9 [DOI] [PubMed] [Google Scholar]
- 21. Tatsumi Y., Yokoo M., Senda H., Kakehi K. 2002. Therapeutic efficacy of topically applied KP-103 against experimental tinea unguium in guinea pigs in comparison with amorolfine and terbinafine. Antimicrob. Agents Chemother. 46:3797–3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Torres-Rodríguez J. M., Dronda M. A., Rossell J., Madrenys N. 1992. Incidence of dermatophytoses in rabbit farms in Catalonia, Spain, and its repercussion on human health. Eur. J. Epidemiol. 8:326–329 [DOI] [PubMed] [Google Scholar]
- 23. Tosti A., Hay R., Arenas-Guzmán R. 2005. Patients at risk of onychomycosis-risk factor identification and active prevention. J. Eur. Acad. Dermatol. Venereol. 19(Suppl. 1):13–16 [DOI] [PubMed] [Google Scholar]
- 24. Van Hoogdalem E. 1997. Nail penetration of the antifungal agent oxiconazole after repeated topical application in healthy volunteers, and the effect of acetylcysteine. Eur. J. Pharm. Sci. 5:119–127 [Google Scholar]
- 25. Van Rooij P., Detandt M., Nolard N. 2006. Trichophyton mentagrophytes of rabbit origin causing family incidence of kerion: an environmental study. Mycoses 49:426–430 [DOI] [PubMed] [Google Scholar]
- 26. Weitzman I., Summerbell R. C. 1995. The dermatophytes. Clin. Microbiol. Rev. 8:240–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zaias N. 1972. Onychomycosis. Arch. Dermatol. 105:263–274 [PubMed] [Google Scholar]