Table 2.
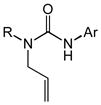
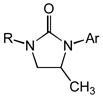
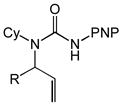
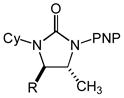
Substrate scope of the intramolecular hydroamination of N-allylic ureas catalyzed by a 1:1 mixture of (P1)AuCl and AgPF6 (1 mol %) in CHCl3 at room temperature (PNP = 4-C6H4NO2, PMP = 4-C6H4OMe).
| entry | substrate | product | time (h) | yield[a] | dr[b] |
|---|---|---|---|---|---|
|
|
||||
| 1[c] | 1b (R = Me; Ar = PNP) | 2b | 48 | 92 | — |
| 2[d] | 1c (R = Ph; Ar = PNP) | 2c | 15 | 93 | — |
| 3[e] | 1d (R = Me; Ar = Ph) | 2d | 72 | 86 | — |
| 4 | 1e (R = Ph; Ar = PMP) | 2e | 16 | 97 | — |
| 5 | 1f (R = Cy; Ar = Ph) | 2f | 30 | 92 | — |
|
|
||||
| 6 | 3a (R = Me) | 4a | 15 | 100 | 50:1 |
| 7 | 3b (R = i-Pr) | 4b | 16 | 93 | 50:1 |
| 8 | 3c (R = CH2OBn) | 4c | 16 | 98 | 50:1 |
| 9 | 3d (R = CH2OAc) | 4d | 16 | 98 | 50:1 |
| 10 |
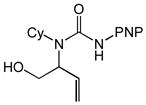 5 |
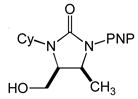 6 |
16 | 97 | 3.7:1 |
Isolated yields of >95% purity.
Determined by 1H NMR analysis of the crude reaction mixture..
Catalyst loading = 5 mol %.
Reaction temperature = 60 °C.
Catalyst loading = 10 mol %.
