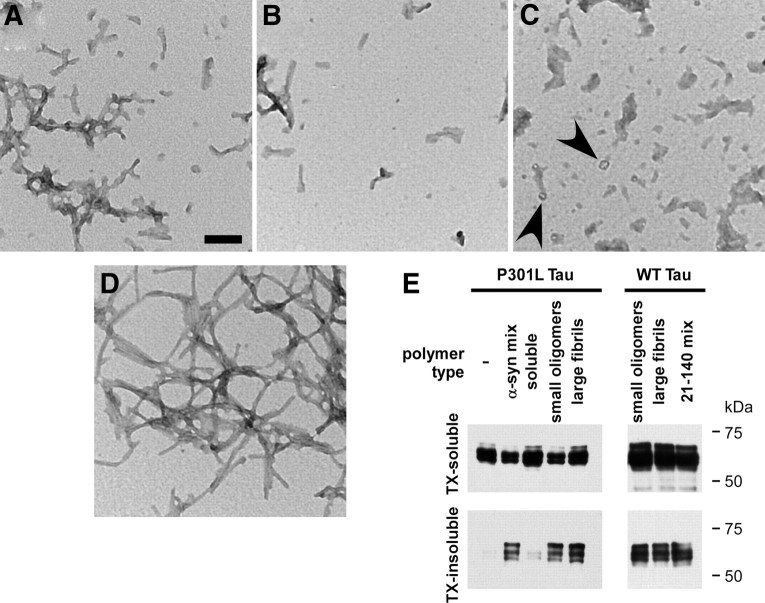
Figure 5.
Polymer subpopulations of fibrillized, recombinant α-syn can promote cellular tau aggregation. Recombinant full-length or 21–140 α-syn was prefibrillized. Before sonication, polymers of full-length α-syn were separated by centrifugation as described in Materials and Methods, and all populations were subjected to sonication. A–D, Ultrastructural electron microscopic analyses were performed on full-length α-syn that had not been separated by centrifugation (mix) (A), small α-syn oligomers (B), large α-syn fibrils (C), or 21–140 α-syn mix (D). Small circular structures were observed in large α-synuclein fibril condition (C, arrowheads). E, Cells expressing WT or P301L tau were treated with each of these polymer types at a final concentration of 1 μm. Cells were harvested 72 h after transfection and biochemical cellular fractionation was performed, separating the Triton X-100 (TX)-soluble from the TX-insoluble fraction. No differences were noted in aggregation propensity between these polymer types, whereas soluble, recombinant, nonfibrillized α-syn did not promote cellular tau aggregation.