Abstract
Paracoccidioidomycosis (PCM) is the most prevalent systemic mycosis in Latin America. It is caused by the dimorphic fungus Paracoccidioides brasiliensis. The immunodiffusion (ID) test is one of the most widely used techniques for PCM serologic diagnosis due to the simplicity and low costs of its execution. However, it requires trained and qualified people to execute it. The purpose of this study was to evaluate a latex particle agglutination (LA) test for the detection of anti-P. brasiliensis antibodies by using pooled crude exoantigens from the fungus. Fifty-one serum samples obtained from patients with PCM were tested. Positivity was observed in 84% (43/51) of these patients, and the agglutination patterns varied from small clumps with a cloudy background to large clumps with a clear background. The antibody titer reactivity ranged from 1:2 to 1:64. Cross-reactivity was observed in sera from patients with aspergillosis, histoplasmosis, and nonfungal disease. Serum samples obtained from healthy donors were not reactive. The sensitivity and specificity of the LA test were 84% and 81%, respectively. When comparing the LA test with the double-immunodiffusion test, we found an agreement of 92%. Further work is needed to improve the performance of the LA assay before it can be proposed as a reliable diagnostic tool, mainly in laboratories with little infrastructure.
INTRODUCTION
Paracoccidioides brasiliensis is the etiologic agent of paracoccidioidomycosis (PCM), a thermally dimorphic fungus that grows in a mycelial form at room temperature and in a budding yeast form at 35 to 37°C in host tissue or when cultured at 37°C (3). The disease is limited to Latin American countries, and the most important regions of endemicity are found in Brazil, Colombia, and Venezuela (14). In Brazil, PCM is considered the eighth most common cause of death among infectious and parasitic chronic diseases, surpassing leishmaniasis, with a mortality rate of 1.45 per million population (4).
Conidia of P. brasiliensis act as infectious propagules which are inhaled into the lungs, where transformation to the pathogenic yeast form occurs (10). PCM exhibits a wide spectrum of clinical and pathological manifestations, ranging from benign and localized forms to severe disseminated disease (11). In most cases, the infection is restricted primarily to the lungs but can spread to other organs (16). The disease presents two major clinical forms: (i) the acute or subacute form (juvenile type), with severe involvement of the mononuclear phagocyte system, and (ii) the chronic form (adult type), with slow evolution and involvement of one or more organs, usually the lungs (7).
The definitive diagnosis of PCM is usually made by visualization or isolation of the fungus from the lesions. Serologic tests appear to offer a means to achieve an early diagnosis of the disease (1, 5). Detection of antibodies in serum has been one of the main tools for the diagnosis of PCM and may be useful to monitor its evolution and its response to treatment. Among the different serologic techniques, the double-immunodiffusion (ID) test is the most commonly used and presents sensitivity values that vary from 80 to 95% (12). However, this technique is feasible only in reference laboratories due to the necessity of having special reagents and experienced people to conduct the test. The development of a simpler and less expensive methodology is greatly needed by laboratories with little infrastructure, especially in poor countries with areas where the disease is endemic. Such a test could contribute to the diagnosis and the screening of PCM, mainly in hospital and public health laboratories, and thereby expand diagnoses of this infection, which affects individuals in their most productive period of life, especially male adults who live in rural areas.
Investigators have shown the latex particle agglutination (LA) test to be a valuable tool for the diagnosis of various diseases, including fungal infections (9, 15). In 1978, Restrepo and Moncada (15), using an LA test with a crude exoantigen prepared from a pool of three strains of P. brasiliensis (B 339, B 341, C 81) for the detection of antibodies, showed that the maximum sensitivity value of the assay was 69.5%, while the specificity value varied from 18.5% to 46.8%.
In order to improve the serological parameters of the agglutination method for the diagnosis of PCM, the present study had the objectives of producing a rapid LA test using pooled crude exoantigen of P. brasiliensis and evaluating its possible use in the serological diagnosis of this mycosis, as well as of comparing results with those of the ID test, which has already been established as a diagnostic tool for PCM.
MATERIALS AND METHODS
Sera and patients.
Fifty-one serum samples obtained from patients with active PCM (47 males and 4 females ranging from 15 to 75 years of age) were included in the study. Six patients presented with the acute form and 45 patients the chronic form of the disease. All of them exhibited clinical and laboratory signs of the disease, such as central nervous and pulmonary system involvement, mucosal or mucocutaneous lesions, and increased specific-antibody levels. The diagnosis was confirmed either by direct examination of biological fluids (n = 4), by serological immunodiffusion tests (n = 25), or by both tests (n = 22). Eleven serum samples from patients with histoplasmosis (HP), 15 from patients with aspergillosis (ASP), and 49 from patients with nonfungal disease (patients presenting bacterial infections) were used. On the other hand, 20 serum specimens from healthy individuals with no history of pulmonary disease were also studied. These sera were termed normal human sera (NHS) and were individually tested and used as negative controls. All serum specimens were divided into aliquots and were stored at −20°C.
Fungal strains and crude exoantigen production.
Three isolates of P. brasiliensis (Pb34, Pb113, and PbIOC), obtained from the culture collection of Evandro Chagas Institute, were selected for this study. The isolates were initially grown on Sabouraud medium slant tubes for 3 days at 35°C. The growth (consisting entirely of yeast cells) was collected from at least 10 tubes, yielding an inoculum of approximately 2 × 106 cells. These cells were inoculated into 500-ml Erlenmeyer flasks containing 100 ml of yeast extract-peptone-dextrose (YEPD) broth (Difco, Sparks, MD). This culture was incubated for 3 days at 35°C on a gyratory shaker at 50 rpm (Fanem, São Paulo, Brazil). The growth obtained was transferred to 1,800-ml Fernbach flasks containing 500 ml of YEPD broth. The flasks were then incubated as described above for 7 more days. The culture was killed with thimerosal (0.2 g/liter). Supernatant fluids were collected following paper filtration, concentrated under vacuum at 45°C, and dialyzed against distilled water. After dialysis, the solution was concentrated again (6).
The protein content was measured by the Bradford method (2), and the electrophoretic pattern was analyzed by SDS-PAGE (8), followed by silver nitrate staining.
ID test.
Immunodiffusion was performed with pooled crude exoantigen of P. brasiliensis as described previously (5). Each PCM, HP, ASP, and NHS specimen was individually tested against an antigenic preparation from P. brasiliensis strains (Pb34, Pb113, and PbIOC), and the titer of each serum sample was determined.
Preparation of the LA test.
The LA test antigen consisted of a pool of six lots of crude exoantigen adjusted to a concentration of 400 μg/ml and was used to coat the test latex particles. A 1% suspension of latex polystyrene beads (diameter, 0.8 μm; Sigma, St. Louis, MO) in carbonate-bicarbonate-buffered saline solution (pH 9.2) was sensitized by mixing the beads with pooled crude exoantigen of P. brasiliensis at 4°C overnight before the addition of bovine serum albumin (BSA) to a final concentration of 0.1% (wt/vol). The optimal solution for use was the one that produced a clear agglutination with the sera of experimentally infected animals and with positive-control sera from patients with PCM. The latex particles (1% suspension) were coated primarily with pooled crude exoantigen (400 μg/ml). The LA test was performed by placing 25 μl of the latex suspension on a dark slide. A total of 25 μl of serum was added to the latex particles. After the reagents were mixed, the slide was gently agitated in a Kline agitator for 5 min. Negative-control latex consisted of 25 μl of latex solution added to 25 μl of saline solution. Samples were considered positive for P. brasiliensis antibodies when agglutination (clumping) was observed. The grading of agglutination was defined as negative when the suspension had a fine granular background or a milky aspect with the absence of agglutination, a one plus (1+) suspension had small clumping against a cloudy background, a two plus (2+) suspension had small to moderately sized clumps against a slightly cloudy background, a three plus (3+) suspension presented moderately sized and large clumps against a clear background, and a four plus (4+) suspension had large clumps with a very clear background. After the screening test of the undiluted serum samples, if the agglutination grade was 1+ or higher, the reaction mixtures were titrated (1:2 through 1:1,024).
Statistical analysis.
Receiver operating characteristic (ROC) curve analysis was performed to determine the cutoff for positivity that would give the optimal sensitivity and specificity. Agreement was calculated by the following formula: (A + D)/(A + B + C + D), where A is a specimen with positive results by both LA and ID, B is a specimen with a positive result by LA but a negative result by ID, C is a specimen with a negative result by LA but a positive result by ID, and D is a specimen with negative results by both assays.
RESULTS
Immunodiffusion and SDS-PAGE analysis of pooled crude exoantigen.
As shown by the immunodiffusion (ID) test, the P. brasiliensis pooled crude exoantigen reacted positively up to the dilution 1:16. SDS-PAGE exoantigen showed at least 12 components (15 to 180 kDa) following silver staining. The main P. brasiliensis antigenic determinants gp43 and gp70 were observed.
Detection of antibodies by the ID and LA tests.
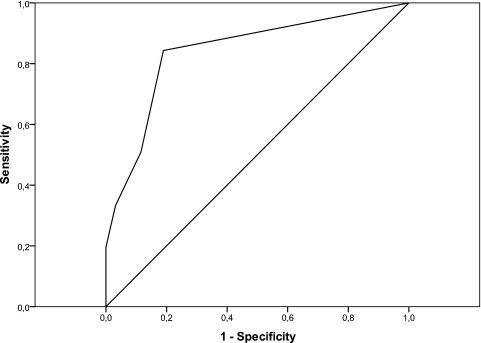
Serum specimens from 51 cases of PCM and 95 controls, including 26 cases with fungal infection (HP, ASP), 20 cases of NHS, and 49 cases of nonfungal diseases were tested simultaneously by ID and LA using a pooled crude exoantigen (Table 1). Among these 51 PCM sera, 47 (92%) were positive and 4 (8%) were not reactive by the ID test. The serum titers obtained by the ID test ranged from 1:2 to 1:1,024 (Table 2). Five PCM sera reacted only with nondiluted serum. ROC analysis identified the optimal cutoff for positivity to be the agglutination pattern equivalent to one plus (1+). The sensitivity and specificity of the ID test were 92% and 100%, respectively. The LA test sensitivity was 84%, the specificity was 81%, and the area under the curve was 0.841 (Fig. 1). The LA and ID tests presented an agreement of 92%.
Table 1.
Detection by the ID and LA tests of anti-P. brasiliensis antibodies in sera from patients with PCM or other mycoses and in sera from healthy individuals
| Patient group | No. of specimens with ID test result: |
Total no. of specimens | No. of specimens with LA test result: |
Total no. of specimens | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||
| PCM | ||||||
| Acute | 6 | 0 | 6 | 6 | 0 | 6 |
| Chronic | 41 | 4 | 45 | 37 | 8 | 45 |
| Unifocal | 19 | 3 | 22 | 17 | 5 | 22 |
| Multifocal | 22 | 1 | 23 | 20 | 3 | 23 |
| Histoplasmosis | 0 | 11 | 11 | 3 | 8 | 11 |
| Aspergillosis | 0 | 15 | 15 | 4 | 11 | 15 |
| Nonfungal diseases | 0 | 49 | 49 | 11 | 38 | 49 |
| NHSa | 0 | 20 | 20 | 0 | 20 | 20 |
χ2 = 42,759; P < 0.0001, PCM and NHS.
Table 2.
Reactivities of 51 PCM patient serum samples by the ID and LA tests
| Serum | ID |
LA |
||
|---|---|---|---|---|
| No. of specimens | % positive | No. of specimens | % positive | |
| Not diluted | 5 | 10 | 16 | 32 |
| 1:2 | 10 | 19 | 5 | 10 |
| 1:4 | 13 | 25 | 3 | 6 |
| 1:8 | 7 | 14 | 5 | 10 |
| 1:16 | 4 | 8 | 4 | 8 |
| 1:32 | 2 | 4 | 8 | 15 |
| 1:64 | 4 | 8 | 2 | 4 |
| >1:64a | 2 | 4 | 0 | 0 |
| Nonreactive | 4 | 8 | 8 | 15 |
| Total | 51 | 100 | 51 | 100 |
One sample corresponded to a dilution of 1:128 and other to 1:1,024.
Fig. 1.
ROC curve depicting assay sensitivity and specificity based upon testing sera from 51 patients with PCM and 95 controls, including patients with histoplasmosis, aspergillosis, and nonfungal diseases and healthy subjects. With a cutoff of one plus (1+), the sensitivity is 84% and the specificity is 81%.
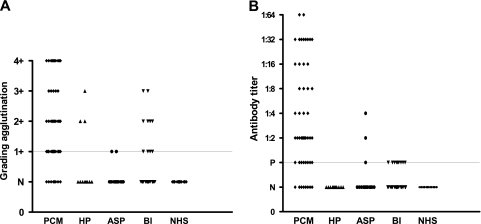
In the LA test, the cutoff value was determined to be one plus (1+) and anti-P. brasiliensis antibodies were detected in 43 out of 51 (84%) serum samples; false-negative results were observed in 8 (16%) samples. Figure 2 shows the results obtained when patients' sera were classified according to the screening results and to the titration procedures. Figure 2A shows the results ranging from 1+ to 4+ for nondiluted sera. Figure 2B shows antibody titers ranging from 1:2 to 1:64 after titration.
Fig. 2.
Detection of anti-P. brasiliensis antibodies by the LA test in the sera of 51 patients with PCM and 95 controls, including patients with histoplasmosis (HP), aspergillosis (ASP), and nonfungal diseases (bacterial infection [BI]) and healthy subjects (NHS). (A) Screening test of the undiluted sera. (B) The titer is reported as the highest dilution showing a 2+ or greater reaction. P, nondiluted sera; N, negative. The long, fine line represents the cutoff point (1+).
The ID and LA tests were able to detect anti-P. brasiliensis antibodies in the acute-PCM form. Of the sera collected from patients with the chronic form, four presented negative results (1 chronic multifocal form, 3 chronic unifocal forms), whereas in the LA test, eight serum samples presented negative results (3 chronic multifocal forms, 5 chronic unifocal forms) (Table 1).
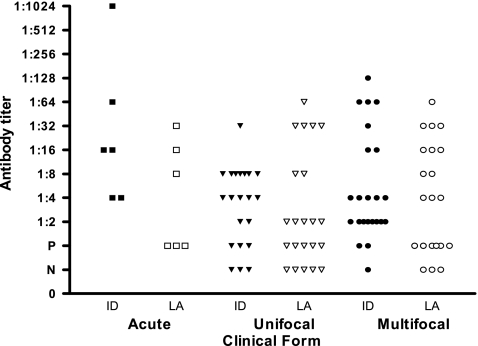
In the ID test, serum samples from patients with the acute form of PCM showed antibody titers ranging from 1:4 to 1:1,024. On the other hand, in the LA test, antibody levels ranged from 1:8 to 1:32. Considering the chronic form, antibody titers varied from 1:2 to 1:128 by the ID test and from 1:2 to 1:64 by the LA test. Figure 3 shows the results obtained when patients' sera were separated according to the different clinical forms. Four sera negative by the ID test were also negative by the LA test. The antibody titers observed in the ID test were higher than those observed in the LA test (Fig. 3).
Fig. 3.
Comparative antibody titers obtained by the ID and LA tests, grouped by PCM clinical form. ID, immunodiffusion; LA, latex agglutination; P, nondiluted sera; N, negative.
A total of 95 heterologous serum samples were tested by the ID and LA tests, including samples from patients with histoplasmosis (n = 11), patients with aspergillosis (n = 15), healthy donors (n = 20), and patients with nonfungal disease (n = 49). In the ID test, all non-PCM serum samples were negative. However, in the LA test, cross-reactivity was obtained with sera from patients with histoplasmosis (27%; 3/11), aspergillosis (27%; 4/15), and nonfungal disease (22%; 11/49). None of these samples reacted at a titer higher than 1:4. No positive reaction was observed in the sera of healthy donors (Table 1; Fig. 2A and B). There were statistically significant differences when the positive reactions of the LA test serum samples from patients with PCM were compared with those of NHS samples (P < 0.0001) (Table 1), which indicates that the test is able to discriminate patients with the disease from those without the disease.
DISCUSSION
Serological tests are useful tools for the diagnosis of systemic fungal infections. These tests are based either on the detection of serum antibodies against fungal components or on the detection of products of the fungus itself. Their effectiveness depends on the reagents, the antigenic preparation, the methods used, and also on a comprehension of their limitations and a precise interpretation of the results obtained. However, they are not routinely available in most public health services. Only a few research laboratories located in areas where systemic mycosis is considered endemic perform them. Paracoccidioidomycosis is endemic in Brazil; however, it is not a notifiable disease, and its real prevalence and incidence cannot be calculated, which makes it difficult to establish its impact on the different Brazilian states.
Our study evaluated the development of a serologic test using latex particles coated with pooled crude exoantigen of P. brasiliensis. P. brasiliensis is a fungus that grows appropriately in a variety of different media, and we obtained a very good antigenic preparation from yeast filtrate culture grown in YEPD medium broth. This antigenic preparation has demonstrated a high degree of sensitivity, specificity, and reproducibility in the serodiagnosis of paracoccidioidomycosis by immunodiffusion in our laboratory. The pooled crude exoantigen used was obtained after homogenization of equivalent amounts of each lot of antigen, with molecular masses ranging from 15 to 180 kDa.
The sensitivity observed in the LA test was 84.31% (43/51), and the antibody titer ranged from 1:2 to 1:64. Eight patients presented false-negative results in the LA test, while in the ID test only four serum samples were not reactive. This may be attributed to several factors, such as different immune responses by the host, since these results were found only in sera from patients presenting the chronic form of PCM, in which low antibody production in PCM unifocal forms is usually observed (12). On the other hand, it is also possible that the fungus strain infecting these patients had different levels of virulence, which could have influenced the immune response. Another possibility may be the loss of conformational epitopes in the LA test using the pooled crude exoantigen of P. brasiliensis.
Restrepo and Moncada (15) tested four different crude antigenic preparations for PCM diagnosis by LA (mycelial and yeast phases, both treated and not treated with ethanol). The crude yeast filtrate used to sensitize the particles was found to be better than the ethanol-treated yeast filtrate; however, sensitivity reached only 69.5% in the assay. A cause for the difference observed between our study and that of Restrepo and Moncada (15) concerning the sensitivity results can be explained by the chemical nature of the substances present in yeast-phase culture filtrates, which are largely constituted of sugars, such as glucose, galactose, arabinose, and glucosamine (17). This antigenic preparation was employed by Restrepo and Moncada in 1978 (15); however, its composition has lower concentrations of antigenic proteins of interest, and it frequently produces high cross-reactivity with sera of patients with histoplasmosis and aspergillosis, influencing the specificity of the test. We utilized an antigen standardized by de Camargo et al. (6), whose specificity was 81.1%, whereas the assay of Restrepo and Moncada (15) showed a maximum specificity of 46.8% when tested with sera of patients with histoplasmosis.
In our study, detection of antibodies by the ID test reached a sensitivity of 92.16% (47/51), with antibody titers ranging from 1:2 to 1:1,024. However, in the sera of four PCM patients, precipitating antibodies were not detected. A possible reason for these false-negative results may be the presence of low-avidity IgG2 antibodies directed against carbohydrate epitopes, which may interfere with ID tests in PCM patients (13).
Antibody reactivity observed in both the ID and LA tests was higher in PCM patients presenting the acute form of the disease than in those with the chronic form. It is known that the acute form of PCM is generally more severe, resulting in higher titers of antibodies, than its chronic form, with which low antibody titers or, occasionally, titer fluctuation may be detected (12). The lack of reactivity (negative- and low-agglutination patterns [1+]) in the LA tests of sera from patients with proven PCM may be related to the production of low titers of antibodies or to intrinsic characteristics of the patient or the infective strain. Although cross-reactions with serum samples from patients with other mycoses could be observed in this test (aspergillosis [4/15] and histoplasmosis [2/11]), reactivities were less intense and occurred at titers (1:4) lower than those of sera from PCM patients and could be minimized by progressive dilutions of the specimens. We believe that this cross-reactivity will not hinder the use of this diagnostic tool in the detection of antibodies for PCM. On the other hand, the fungus probably shares common antigenic determinants or similar determinants of related chemical structures, which could explain cross-reaction. Travassos et al. (18) reported that, in general, specific reactions are obtained when antigen is in a solution similar to that in the ID test and that fixing antigen onto a solid-phase substrate increases the number of nonspecific reactions. Nevertheless, other assays using latex particles coated with pooled crude exoantigens pretreated with sodium metaperiodate (13) and serum samples pretreated with 2-β-mercaptoethanol must be conducted to eliminate cross-reactivity. Such a procedure will optimize the chances of accurately identifying true-positive LA test results in serum samples.
One of the main problems in the serodiagnosis of PCM based on antibody detection is the cross-reaction obtained with sera from patients with other mycoses, mainly histoplasmosis and aspergillosis (12, 15). On the other hand, lack of reactivity or low antibody titers have been a very common problem in our region. With the cutoff set at 1+ and the subsequent titration of the samples, some patients with proven PCM were serologically classified as negative by the LA test. However, some of these patients also had negative results by the ID test. We believe that the format of the test and the patient's immune status may have contributed to such results. However, it is possible that other factors (prozone, the presence of rheumatoid factor, and other serum macroglobulins) interfere with the performance of the test, which means that new studies must be conducted in order to better evaluate this serological pattern.
The ID test is still the gold standard serological test used in the diagnosis of PCM. It shows sensitivity and specificity of approximately 95 to 100% (5, 13). It is an easy-to-perform simple test whose results can be obtained within 4 days; it is used mainly in research institutes, because it requires equipment and qualified laboratory staff for the production of antigens. These antigens show great variability, making it very difficult to standardize the diagnostic techniques in different laboratories.
The LA test used for other fungal pathologies has the advantage of being rapid and simple, and it is not affected by anticomplement activity (9). Another important advantage of this test is its stability. Sensitized latex beads were found to be able to retain similar agglutination sensitivities after storage at 4°C for at least 4 months (data not shown). By following the precise procedure in the test, the assay can be useful as a screening test in hospitals and public health laboratories, where the use of a battery of tests or more complex procedures is not feasible. The rapid agglutination-based latex particle assay developed in this study showed that it is crucial to examine the effect of pretreatment of antigen and serum samples on the performance of the assay to increase sensitivity and specificity and to minimize or eliminate cross-reactivity with sera of patients with other mycoses before the assay can be suggested as an additional reliable diagnostic tool in the qualitative presumptive diagnosis of PCM and for use in follow-up studies in areas where the disease is endemic, mainly in laboratories with poor infrastructure.
ACKNOWLEDGMENTS
We thank André Monteiro Diniz for proofreading the manuscript. We thank all anonymous referees, whose comments have significantly improved the manuscript.
This study was supported by grants from CNPq (process number 471106/2007-0).
Footnotes
Published ahead of print on 16 February 2011.
REFERENCES
- 1. Albuquerque C. F., Marques da Silva S. H., Camargo Z. P. 2005. Improvement of the specificity of an enzyme-linked immunosorbent assay for diagnosis of paracoccidioidomycosis. J. Clin. Microbiol. 43:1944–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bradford M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 3. Brummer E., Castaneda E., Restrepo A. 1993. Paracoccidioidomycosis: an update. Clin. Microbiol. Rev. 6:89–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coutinho Z. F., et al. 2002. Paracoccidioidomycosis mortality in Brazil (1980–1995). Cad. Saúde Pública 18:1441–1454 [DOI] [PubMed] [Google Scholar]
- 5. de Camargo Z. P., Unterkircher C., Campoy S. P., Travassos L. R. 1988. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion tests. J. Clin. Microbiol. 26:2147–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Camargo Z. P., Berzaghi R., Amaral C. C., Silva S. H. 2003. Simplified method for producing Paracoccidioides brasiliensis exoantigens for use in immunodiffusion tests. Med. Mycol. 41:539–542 [DOI] [PubMed] [Google Scholar]
- 7. Franco M. F., et al. 1987. Paracoccidioidomycosis: a recently proposed classification of its clinical forms. Rev. Soc. Bras. Med. Trop. 20:129–132 [DOI] [PubMed] [Google Scholar]
- 8. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 9. Martins T. B., Jaskowski T. D., Mouritsen C. L., Hill H. R. 1995. Comparison of commercially available enzyme immunoassay with traditional serological tests for detection of antibodies to Coccidioides immitis. J. Clin. Microbiol. 33:940–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McEwen J. G., Bedoya V., Patiño M. M., Salazar M. E., Restrepo A. 1987. Experimental murine paracoccidioidomycosis induced by the inhalation of conidia. J. Med. Vet. Mycol. 25:165–175 [DOI] [PubMed] [Google Scholar]
- 11. Mendes R. P. 1994. The gamut of clinical manifestations, p. 233–258 In Franco M. F., Lacaz C. S., Restrepo A., Del Negro G. (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, FL [Google Scholar]
- 12. Mendes-Giannini M. J., Del Negro G. B., Siqueira A. M. 1994. Serodiagnosis, p. 345–363 In Franco M., Lacaz C. S., Restrepo A., Del Negro G. (ed.), Paracoccidioidomycosis. CRC Press, Boca Raton, FL [Google Scholar]
- 13. Neves A. R., Mamoni R. L., Rossi C. L., Camargo Z. P., Blotta M. H. S. L. 2003. Negative immunodiffusion test results obtained with sera of paracoccidioidomycosis patients may be related to low-avidity immunoglobulin G2 antibodies directed against carbohydrate epitopes. Clin. Diagn. Lab. Immunol. 5:802–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Restrepo A. 1985. The ecology of Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 23:323–334 [PubMed] [Google Scholar]
- 15. Restrepo A., Moncada L. H. 1978. Una prueba de látex em lamina para el diagnostico de la paracoccidioidomicosis. Bol. Oficina Sanit. Panam. 84:520–532 [PubMed] [Google Scholar]
- 16. Restrepo-Moreno A. 2003. Paracoccidioidomycosis, p. 328–345 In Dismukes W. E., Pappas P. G., Sobel J. D. (ed.), Clinical mycology. Oxford University Press, New York, NY [Google Scholar]
- 17. Restrepo-Moreno A., Schneidau J. D., Jr 1967. Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J. Bacteriol. 93:1741–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Travassos L. R., Taborda C. P., Iwai I. K., Cunha-Neto E., Puccia R. 2004. The gp43 from Paracoccidioides brasiliensis: a major diagnostic antigen and vaccine candidate, p. 279–296 In Domer J. E., Kobayashi G. S. (ed.), The mycota, vol. XII Human fungal pathogens Springer-Verlag, Berlin, Germany [Google Scholar]