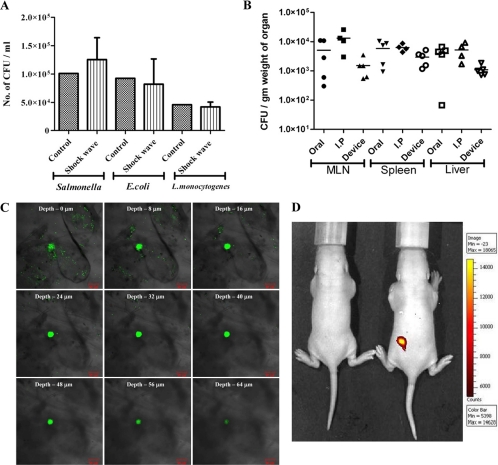
Fig. 3.
In vitro and in vivo testing of the device. (A) Gram-negative (Salmonella and E. coli) and Gram-positive (Listeria) bacterial cultures were placed in the cavity (without a discharge hole) and subjected to micro-shock waves to check the viability of bacteria using the device. The control bacterial culture was not subjected to micro-shock wave treatment. (B) Salmonella enterica serovar Typhimurium vaccine strain pmrG-HM-D (DV-STM-07) was administered to mice using the device, and the mice were sacrificed and dissected after 3 days to check the entry of bacteria in secondary lymphoid organs like mesenteric lymph nodes (MLN), spleen, and liver. In the control mice, DV-STM-07 was delivered by the oral or intraperitoneal (I.P) route. Each symbol represents the value for an individual mouse. The short black line shows the mean value for the group (5 mice in each group). (C) Confocal images (xyz scanning) showing the penetration of fluorescent beads delivered to the abdominal region of mouse skin using the device. (D) Fluorescent beads were delivered to the dorsal side of mice using the device, and a biofluorescence image showing the presence of fluorescent beads is shown. Min, minimum; Max, maximum.