Abstract
Before use of the pneumococcal conjugate vaccine PCV7 became widespread in Turkey, 202 invasive pneumococcus isolates were analyzed. The most common serotypes were 19F and 6B. In children ≤2 years of age, the potential coverage rate of PCV7 was 69.5%. The most frequent non-PCV7 serotypes were 19A, 3, 1, 6A, and 8.
TEXT
Streptococcus pneumoniae (pneumococcus) is the leading cause of severe pneumonia in children across the world (26). The World Health Organization (WHO) estimates that about 700,000 to 1 million children die of pneumococcal disease every year, most of whom are young children in developing countries (30). In 2007, WHO recommended that pneumococcal conjugate vaccine PCV7 be included in national immunization programs (NIP) (30). As of May 2009, PCV7 was licensed in over 90 countries and included in the NIP of 36 countries worldwide, including Turkey (24). PCV7 currently represents the only PCV with proven global effectiveness against invasive pneumococcal disease (IPD) (1, 5, 8, 20, 22, 29). Recently, two more PCVs have been introduced; PCV10 covers serotypes 1, 5, and 7F in addition to the PCV7 serotypes, and PCV13 covers serotypes 3, 6A, and 19A in addition to the PCV10 serotypes (14).
The percentage of circulating serotypes causing IPD that are covered by PCV7 ranged from 60% to 80% for European children <2 years of age (15). In the United States, population-based and laboratory-based surveillance data indicated that overall IPD rates among children <5 years of age were 77% lower (approximately 13,000 fewer cases) in 2005 than in the years prior to introduction of the vaccine (1998 to 1999) (2).
In a study conducted in Turkey between the years 2001 and 2004, the proportion of potentially preventable cases was found to be the highest (63%) in the age group of 0 to 24 months (31). From November 2008, PCV7 has been included in the NIP in Turkey for children aged <2 years (17). Wide-scale use of PCV7 has been shown to change the circulating serotypes in a population, and nonvaccine serotypes like 19A appear in a higher percentage of the remaining viral burden that can cause IPD (16, 20, 23, 28). Therefore, surveillance of pneumococcal diseases on the basis of serotypes is essential, not only to learn about the current serotype pattern but also to observe the effectiveness of PCVs by following possible serotype changes in the population after vaccine introduction. In several studies, regional and temporal variations in serotype distribution were reported after PCV usage (6, 7, 10–12). In this study, we aimed to identify serotypes of pneumococcal strains causing IPD in children in Turkey during the time period when PCV7 was newly included in the country's NIP.
S. pneumoniae strains were consecutively isolated at 15 different health centers in Turkey, serving about 55% of the country's population, between July 2008 and February 2010. Approval was obtained from the ethics committee of the coordinator center, in accordance with local regulations. The pneumococci were isolated as a part of the routine clinical diagnostic practice, from blood, cerebrospinal fluid, and other body fluids of pediatric cases (≤18 years) with IPD. Penicillin susceptibility testing was performed according to the guidelines of the Clinical and Laboratory Standards Institute (3). Serotyping was performed by the Quellung reaction using serotype-specific antisera (Statens Seruminstitut, Copenhagen, Denmark).
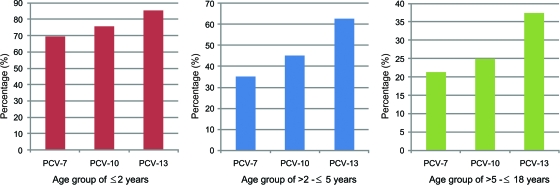
The most common serotypes were 19F, 6B, 4, 14, 19A, 3, 23F, and 1, in decreasing order (Table 1). During the first 2 years of age, the potential coverage rates of PCV7, PCV10, and PCV13 were 69.5%, 75.8%, and 85.3%, respectively; coverage rates of these vaccines were 35.3%, 45.1%, and 62.7% for the >2 to ≤5-year age group and 21.4%, 25.0%, and 37.5% for the >5 to ≤18-year age group (Fig. 1). The most common serotypes were reported to be 14, 6, 19, 23, and 18 in Western Europe countries in the <18-year age group, 6B, 6A, 14, 5, and 23F in Egypt in the <2-year age group, and 3, 4, 6, 14, and 23 in Saudi Arabia in the <2-year age group (11, 15).
Table 1.
Serotype distribution of invasive S. pneumoniae isolates by age group
| Serotype | No. of isolates per age group |
Total no. of isolates | ||
|---|---|---|---|---|
| ≤2 yr | >2–≤5 yr | >5–≤18 yr | ||
| PCV7 serotypes | ||||
| 19F | 27 | 9 | 3 | 39 |
| 6B | 13 | 1 | 2 | 16 |
| 4 | 9 | 2 | 3 | 14 |
| 14 | 9 | 1 | 2 | 12 |
| 23F | 6 | 2 | 8 | |
| 18C | 1 | 3 | 1 | 5 |
| 9V | 1 | 1 | 2 | |
| Other vaccine serotypes | ||||
| 19A | 6 | 3 | 1 | 10 |
| 3 | 6 | 4 | 10 | |
| 1 | 4 | 2 | 2 | 8 |
| 6A | 3 | 2 | 5 | |
| 5 | 1 | 3 | 4 | |
| 7F | 1 | 1 | ||
| Other serotypes | ||||
| 7A | 1 | 4 | 5 | |
| 8 | 2 | 3 | 5 | |
| 15 | 1 | 1 | 3 | 5 |
| 15C | 2 | 3 | 5 | |
| 2 | 2 | 2 | ||
| 10 | 1 | 1 | 2 | |
| 6 | 1 | 1 | ||
| 17 | 1 | 1 | ||
| 16F | 1 | 1 | ||
| 23A | 1 | 1 | ||
| Not determined | 2 | 4 | 7 | 13 |
| Other | 4 | 11 | 12 | 27 |
| Total | 95 | 51 | 56 | 202 |
Fig. 1.
Vaccine coverage rates for PCV7, PCV10, and PCV13 by age group.
Of 202 invasive isolates, 68 (33.7%) were penicillin nonsusceptible (PNS). Of these PNS isolates, 66.2% (n = 45) were from children ≤2 years of age. The proportions of PNS isolates potentially covered by the PCV7, PCV10, and PCV13 vaccines were 77.8%, 82.2%, and 91.1%, respectively, in the first 2 years of age. The proportion of PNS isolates in children was approximately 11% in Western Europe countries, with the highest proportion (49%) in Spain and the lowest proportion (<1%) in Germany (13). This proportion is about 50% in Egypt and 44% in Saudi Arabia (9).
Our results suggest that the incidence of pneumococcal diseases can be decreased by widespread use of the vaccine in Turkey due to the fact that the potential PCV7 coverage rate is approximately 70% in patients ≤2 years of age, the target population for vaccination. However, gradual serotype replacement is another global fact seen with PCV7 NIP. In the United States, the total IPD burden, which has significantly been decreased by PCV7, seems to have reached a plateau due to the gradual increase of the non-PCV7 serotypes, mainly 19A (20). Similar changes were also reported from European countries after the introduction of PCV7 (27). Greece, a neighboring country running an NIP for PCV7, has also reported 19A as the leading non-PCV7 serotype (V. Syriopoulou et al., Paediatric pneumococcal serotype epidemiology after the introduction of the heptavalent conjugated vaccine in Greece: an interim analysis, poster 261, presented at World Society for Pediatric Infectious Diseases, 2009). In our study, serotypes 19A and 3 were the most frequent non-PCV7 serotypes, totaling almost 10%. Thus, our results appear to show a potential background for a similar serotype replacement due to the national PCV7 vaccination in Turkey.
Penicillin has been universally effective in treating IPD, but the emergence of resistant strains is causing problems (18, 21). The prevalence of PNS pneumococci appears to be on the rise in Turkey (25). Our results showed that most of the PNS strains (66.2%) were isolated from patients ≤2 years old and PCV7 vaccination led to a considerable coverage in PNS IPD isolates (77.8% for the ≤2-year age group). With the PCV7 NIP, we may now have a considerable decrease in PNS IPD cases in Turkey, as has previously been seen in the United States and in Europe (4, 15).
We believe that ongoing surveillance of pneumococcal diseases is essential to closely monitor the disease dynamics after routine use of PCV7 or the other PCVs.
Acknowledgments
This study was supported by an unrestricted research grant, 0887X1-4439, from Wyeth-Turkey.
Footnotes
Published ahead of print on 20 April 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Black S., et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19:187–195 [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC) 2008. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction—eight states, 1998-2005. MMWR Morb. Mortal. Wkly. Rep. 57:144–148 [PubMed] [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2009. Performance standards for antimicrobial disk susceptibility tests, 10th ed. Approved standard. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. de la Campa A. G., et al. 2009. Changes in fluoroquinolone-resistant Streptococcus pneumoniae after 7-valent conjugate vaccination, Spain. Emerg. Infect. Dis. 15:905–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durando P., et al. 2009. Universal childhood immunization against Streptococcus pneumoniae: the five-year experience of Liguria Region, Italy. Vaccine 27:3459–3462 [DOI] [PubMed] [Google Scholar]
- 6. Feikin D. R., Klugman K. P. 2002. Historical changes in pneumococcal serogroup distribution: implications for the era of pneumococcal conjugate vaccines. Clin. Infect. Dis. 35:547–555 [DOI] [PubMed] [Google Scholar]
- 7. Feikin D. R., et al. 2005. Increased prevalence of pediatric pneumococcal serotypes in elderly adults. Clin. Infect. Dis. 41:481–487 [DOI] [PubMed] [Google Scholar]
- 8. Grijalva C. G., et al. 2007. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the U. S. A.: a time-series analysis. Lancet 369:1179–1186 [DOI] [PubMed] [Google Scholar]
- 9. Hausdorff W. P., Hajjeh R., Al-Mazrou A., Shibl A., Soriano-Gabarro M., the Middle East & North Africa Vaccine-Preventable Diseases Regional Advisory Group 2007. The epidemiology of pneumococcal, meningococcal, and Haemophilus disease in the Middle East and North Africa (MENA) region—current status and needs. Vaccine 25:1935–1944 [DOI] [PubMed] [Google Scholar]
- 10. Imöhl M., Reinert R. R., van der Linden M. 2009. Adult invasive pneumococcal disease between 2003 and 2006 in North-Rhine Westphalia, Germany: serotype distribution before recommendation for general pneumococcal conjugate vaccination for children <2 years of age. Clin. Microbiol. Infect. 15:1008–1012 [DOI] [PubMed] [Google Scholar]
- 11. Imöhl M., Reinert R. R., van der Linden M. 2010. Regional differences in serotype distribution, pneumococcal vaccine coverage, and antimicrobial resistance of invasive pneumococcal disease among German federal states. Int. J. Med. Microbiol. 300:237–247 [DOI] [PubMed] [Google Scholar]
- 12. Jacobs M. R., Good C. E., Bajaksouzian S., Windau A. R. 2008. Emergence of Streptococcus pneumoniae serotypes 19A, 6C, and 22F and serogroup 15 in Cleveland, Ohio, in relation to introduction of the protein-conjugated pneumococcal vaccine. Clin. Infect. Dis. 47:1388–1395 [DOI] [PubMed] [Google Scholar]
- 13. Jefferson T., Ferroni E., Curtale F., Giorgi Rossi P., Borgia P. 2006. Streptococcus pneumoniae in Western Europe: serotype distribution and incidence in children less than 2 years old. Lancet Infect. Dis. 6:405–410 [DOI] [PubMed] [Google Scholar]
- 14. Johnson H. L., et al. 2010. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the Pneumococcal Global Serotype Project. PLoS Med. 7:e1000348 doi: 10.1371/journal.pmed.1000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kyaw M. H., et al. 2006. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 354:1455–1463(Erratum: 355:638.) [DOI] [PubMed] [Google Scholar]
- 16. Mahjoub-Messai F., et al. 2009. Population snapshot of Streptococcus pneumoniae serotype 19A isolates before and after introduction of seven-valent pneumococcal vaccination for French children. J. Clin. Microbiol. 47:837–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ministry of Health of Turkey General Directorate of Primary Health Care Services 1 December 2010, accession date Genişletilmiş Bağışıklama Programı Genelgesi. http://www.saglik.gov.tr/TR/belge/1-8187/genisletilmis-bagisiklama-programi-genelgesi-2009.html
- 18. Mokaddas E. M., Rotimi V. O., Albert M. J. 2008. Implications of Streptococcus pneumoniae penicillin resistance and serotype distribution in Kuwait for disease treatment and prevention. Clin. Vaccine Immunol. 15:203–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reference deleted.
- 20. Pilishvili T., et al. 2010. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 201:32–41 [DOI] [PubMed] [Google Scholar]
- 21. Reinert R. R. 2004. Pneumococcal conjugate vaccines—a European perspective. Int. J. Med. Microbiol. 294:277–294 [DOI] [PubMed] [Google Scholar]
- 22. Roche P. W., et al. 2008. Invasive pneumococcal disease in Australia, 2006. Commun. Dis. Intell. 32:18–30 [PubMed] [Google Scholar]
- 23. Rose M., Zielen S. 2009. Impact of infant immunization programs with pneumococcal conjugate vaccine in Europe. Expert Rev. Vaccines 8:1351–1364 [DOI] [PubMed] [Google Scholar]
- 24. Schranz J. 2009. Pneumococcal conjugate vaccines: what do we know and what do we need? Procedia Vaccinol. 1:189–205 [Google Scholar]
- 25. Sener B., et al. 2007. A survey of antibiotic resistance in Streptococcus pneumoniae and Haemophilus influenzae in Turkey, 2004-2005. J. Antimicrob. Chemother. 60:587–593 [DOI] [PubMed] [Google Scholar]
- 26. United Nations Children's Fund/World Health Organization 1 April 2011, accession date Pneumonia: the forgotten killer of children. http://www.unicef.org/mdg/mortalitymultimedia/Pneumonia_The_Forgotten_Killer_of_Children.pdf
- 27. Varon E., Janoir C., Gutmann L. 2008. Rapport d'activité 2008. Centre National de Référence des Pneumocoques, Créteil, France: http://www.invs.sante.fr/surveillance/cnr/rapports_pneumocoques2008.pdf [Google Scholar]
- 28. Vestrheim D. F., et al. 2010. Indirect effect of conjugate pneumococcal vaccination in a 2+1 dose schedule. Vaccine 28:2214–2221 [DOI] [PubMed] [Google Scholar]
- 29. Vestrheim D. F., et al. 2008. Effectiveness of a 2+1 dose schedule pneumococcal conjugate vaccination programme on invasive pneumococcal disease among children in Norway. Vaccine 26:3277–3281 [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization 2007. Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly. Epidemiol. Rec. 82:93–104 [PubMed] [Google Scholar]
- 31. Yalçin I., et al. 2006. Serotype distribution and antibiotic susceptibility of invasive Streptococcus pneumoniae disease isolates from children in Turkey, 2001-2004. Eur. J. Pediatr. 165:654–657 [DOI] [PubMed] [Google Scholar]