Abstract
PCR-hybridization was compared to culture methods for evaluating suspected blood infections. A total of 231 clinical samples from blood culture bottles that were flagged positive by the BacT/Alert system or were negative 1 week after inoculation were tested. When the PCR-hybridization and culture method results were compared, the positive and negative concordance rates were 99.2% (122/123) and 89.5% (94/105), respectively. Of the negative blood cultures, 10.5% (11/105) were positive by PCR-hybridization. Supplemental testing of negative blood cultures may identify bacterial pathogens that are undetectable by culture methods.
TEXT
Blood culture is currently the gold standard method for identification of bacterial pathogens causing blood infections. Accurate and reliable identification of pathogens is critical so that proper and timely treatment can be initiated. Current culture procedures typically require from several days to a week for final results (1, 14), and incorrect results can occur, with false-positive and false-negative rates generally estimated at 2 to 3% (5, 8). Factors suspected of contributing to false-negative culture results include insufficient blood sample inoculum, empirical or long-term antibiotic use prior to diagnosis, and infection due to fastidious organisms (3). This study compared identification of bacterial pathogens by traditional culture methods with PCR amplification of 16S rRNA genes from cultured blood samples in conjunction with hybridization to species-specific probes on a bead array chip.
Clinical samples from aerobic blood culture bottles (BacT/Alert SA standard aerobic culture media bottle, bioMérieux) were obtained from the clinical microbiology laboratory at the Children's Hospital Los Angeles. All of the clinical samples were obtained from pediatric patients. Generally, pediatric samples were used to inoculate one blood culture bottle, not a “set” of two bottles as is standard protocol for adult patients. Positive cultures were those with a positive result in the BacT/Alert system within 1 week of inoculation, while negative cultures were those with a negative result 1 week after inoculation. A total of 231 blood cultures were selected for the study after they were flagged positive by BacT/Alert or upon a final negative result. After culture results were known, DNA was extracted from 500 μl of blood culture broth in a class II cabinet using an alkaline lysis method (9, 13) and resuspended in a final volume of 500 μl.
A pair of universal primers was selected from the highly conserved region of the 16S rRNA sequence (11). The upstream primer corresponded to C1 region nucleotides 358 to 378 (5′-ACT CCT ACG GGA GGC AGC AGT-3′), and the downstream primer corresponded to C6 region nucleotides 1444 to 1425 (5′-TCA CCG GCC GTG TGT ACA AG-3′).
PCR amplification was performed in 20 μl containing 1 μl of extracted DNA, 18 μl of 1× PCR master mix (10 mM Tris-HCl, pH 8.0, 50 mmol/liter KCl, 0.1% Triton X-100, 3.5 mmol/liter MgCl2, 200 μmol/liter each of PCR grade deoxynucleoside triphosphates [dNTPs]) and 1 μl (5u/μl) of DNA polymerase (Hot Start Taq; Qiagen) using the following cycle parameters: 40 cycles of 95°C for 15 min, 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, followed by 72°C for 8 min. PCR amplification produced a 1,086-bp product representing a portion of the 16S rRNA gene (11).
The 17 most common bacterial pathogens responsible for blood infections occurring over a 1-year period were selected from a clinical laboratory database for analysis by PCR-hybridization (Table 1). Oligonucleotide probes specific for the 16S rRNA genes of each of the 17 pathogens, as well as universal probes for Gram-positive, Gram-negative, and all bacteria, were designed (4, 12; N. Mezokh, K. Podual, and M. Seul, presented at the Association of Molecular Pathology AMP Annual Meeting, Orlando, FL, 16 November 2006), coupled to encoded beads stained with spectrally distinguishable combinations of fluorescent dyes, and embedded onto a bead array chip (2, 6, 10). After array assembly, the color code of each bead within the array was recorded. Labeled PCR amplicon was hybridized to the bead array, followed by analysis of the fluorescence pattern by an image-reading microscope with fluorescence optics and a charge-coupled-device camera (2, 6, 10). Decoded image data were converted into normalized results and displayed as bar graphs. For each probe, normalized results represented probe signal intensity corrected for a negative-control value.
Table 1.
Bacterial species evaluated by PCR-hybridization
| Pathogen no. | Species | Result of tests with known standards (no. positive/total no.)b |
|---|---|---|
| 1 | Staphylococcus aureus | 6/6 |
| 2 | Streptococcus pneumoniae | 2/3a |
| 3 | Enterococcus faecalis | NT |
| 4 | Enterococcus faecium | 2/2 |
| 5 | Staphylococcus epidermidis | 54/55a |
| 6 | Streptococcus pyogenes | 1/1 |
| 7 | Streptococcus agalactiae | 3/4a |
| 8 | Escherichia coli | 12/12 |
| 9 | Pseudomonas aeruginosa | 3/3 |
| 10 | Klebsiella pneumoniae | 6/6 |
| 11 | Klebsiella oxytoca | 2/2 |
| 12 | Proteus sp. (P. mirabilis and P. vulgaris) | NT |
| 13 | Enterobacter cloacae | 11/11 |
| 14 | Enterobacter aerogenes | NT |
| 15 | Haemophilus influenzae | 1/1 |
| 16 | Stenotrophomonas maltophilia | 2/2 |
| 17 | Serratia marcescens | NT |
Negative result was due to a weak hybridization signal; PCR result was positive.
NT, not tested.
Quality control evaluation of the PCR-hybridization method was performed with 111 known microbial isolates representing 13 of the 17 bacterial species to be evaluated and 3 isolates of Candida albicans (Table 1). Among the 108 bacterial isolates tested, 105 were correctly identified. The PCR-hybridization results for the three C. albicans isolates were negative, as expected.
A total of 233 (128 culture-positive and 105 culture-negative) clinical blood culture samples were examined by PCR-hybridization and compared with the matched Bac-T/Alert culture results. Among the 128 positive cultures, 123 contained a single bacterial species and 5 were identified as mixed infections. Of the 123 single infected cultures, 122 (99.2%) demonstrated concordant results by PCR-hybridization (Table 2). The one PCR-hybridization-negative sample, positive for coagulase-negative streptococcus (CONS) by Bac-T/Alert, was positive by PCR, but the species was unidentified due to a weak hybridization signal. Of the 105 Bac-T/Alert culture-negative samples, 94 (89.5%) were also negative by PCR-hybridization. In the 11 (10.5%) culture negative, PCR-hybridization-positive samples, hybridization results identified 9 samples containing CONS. The two remaining samples contained Gram-positive bacteria not represented in the bead-array panel. Of the five mixed infections, PCR-hybridization results were consistent with culture results for three samples (Table 3, samples 1 to 3). For the remaining two samples, described below, PCR-hybridization did not clearly detect one of the bacteria identified by culture.
Table 2.
Comparison of results from blood culture with PCR-hybridization for single infectionsa
| Blood culture result | No. with PCR-hybridization result |
Total no. | % Concordance (95% CI)b | |
|---|---|---|---|---|
| + | − | |||
| + | 122 | 1 | 123 | 99.20 (95.6–99.9) |
| − | 11 | 94 | 105 | 89.50 (82.0–94.7) |
+, positive; −, negative.
Exact binomial.
Table 3.
Bacteria identified by blood culture and PCR-hybridization from mixed infections
| Sample no. | Identification by: |
|
|---|---|---|
| Blood culture | PCR-hybridization | |
| 1 | Group D Enterococcus | E. faecalis |
| CONS | S. epidermidis | |
| 2 | E. cloacae | E. cloacae |
| K. pneumoniae | K. pneumoniae | |
| 3 | E. faecalis | E. faecalis |
| CONS | Staphylococcus epidermidis | |
| Acinetobacter calcoaceticus/baumannii complexa | Gram-negative bacteria (universal probe) | |
| 4b | E. aerogenes | E. aerogenes |
| P. putidaa | Pseudomonas sp. | |
| Stenotrophomonas maltophilia | Stenotrophomonas maltophilia (weak signal) | |
| 5c | E. aerogenes | |
| Pseudomonas sp. | ||
| Stenotrophomonas maltophilia | Stenotrophomonas maltophilia (weak signal) | |
Probe for this bacterial species was not included in the bead array chip.
Same patient as sample 5; blood culture from admission blood sample.
Same patient as sample 4; blood culture after antibiotic therapy.
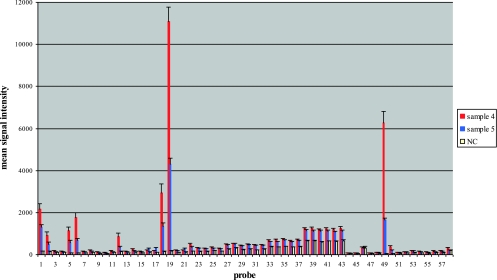
The blood cultures evaluated in this study incidentally included two separate cultures from one patient (Table 3, samples 4 and 5). The first culture was inoculated on admission of the patient before antibiotic therapy was initiated and was positive for Enterobacter aerogenes and Pseudomonas by BacT/Alert (P. putida) and PCR-hybridization (Pseudomonas sp.) (Table 3; Fig. 1). Culture results were also positive for Stenotrophomonas. A second blood culture, inoculated 2 days after admission, was culture negative for E. aerogenes and P. putida, consistent with exposure to antibiotic therapy. In contrast, the PCR-hybridization results for this second sample remained positive for E. aerogenes and Pseudomonas sp. (Fig. 1), consistent with detection of the presence of nonviable bacteria after exposure to antibiotic therapy. The second sample culture results remained positive for Stenotrophomonas, due to treatment with a carbapenem class of antibiotics. Detection of Stenotrophomonas by PCR-hybridization was weak and was recognized only after positive culture results for Stenotrophomonas were obtained.
Fig. 1.
PCR-hybridization bead array results for two samples from one patient. Positive results from the patient's admission sample (sample 4) for normalized values were observed for E. aerogenes (probes 18 and 19) and a Pseudomonas sp. (probe 49). Blood culture results for this sample were positive for E. aerogenes, P. putida, and Stenotrophomonas maltophilia (Table 3). Positive results from the patient's post-antibiotic-therapy sample (sample 5) for normalized values were observed for E. aerogenes (probes 18 and 19) and a Pseudomonas sp. (probe 49). Overall mean signal strength is reduced compared to sample 4 results. Blood culture results for this sample were positive for Stenotrophomonas maltophilia (Table 3). Bacterial species corresponding to probe numbers are shown in Table 4. Results are normalized relative to the signal for the negative control (NC).
Table 4.
Bacterial probes shown in Fig. 1
| Probe no. | Bacterium |
|---|---|
| 1 | Universal |
| 2 | Universal |
| 3 | Gram positive |
| 4 | Gram positive |
| 5 | Gram negative |
| 6 | Gram negative |
| 7 | E. coli |
| 8 | E. coli |
| 9 | Pseudomonas sp. |
| 10 | P. aeruginosa |
| 11 | P. aeruginosa |
| 12 | Klebsiella oxytoca |
| 13 | Klebsiella oxytoca |
| 14 | Klebsiella pneumoniae |
| 15 | Klebsiella pneumoniae |
| 16 | Stenotrophomonas maltophilia |
| 17 | Stenotrophomonas maltophilia |
| 18 | Enterobacter aerogenes |
| 19 | Enterobacter aerogenes |
| 20 | Enterobacter cloacae |
| 21 | Stenotrophomonas maltophilia |
| 22 | Serratia marcescens |
| 23 | Serratia marcescens |
| 24 | Haemophilus influenzae |
| 25 | Haemophilus influenzae |
| 26 | Haemophilus influenzae |
| 27 | Enterobacter sp. |
| 28 | Enterobacter sp. |
| 29 | Enterococcus faecalis |
| 30 | Enterococcus faecalis |
| 31 | Enterococcus faecium |
| 32 | Enterococcus faecium |
| 33 | Streptococcus agalactiae |
| 34 | Streptococcus agalactiae |
| 35 | Streptococcus pyogenes |
| 36 | Streptococcus pyogenes |
| 37 | Streptococcus pneumoniae |
| 38 | Staphylococcus aureus |
| 39 | Staphylococcus aureus |
| 40 | Staphylococcus aureus |
| 41 | Staphylococcus epidermidis |
| 42 | Staphylococcus epidermidis |
| 43 | Staphylococcus epidermidis |
| 44 | E. coli |
| 45 | P. aeruginosa |
| 46 | Streptococcus agalactiae |
| 47 | Streptococcus pyogenes |
| 48 | Pseudomonas sp. |
| 49 | Pseudomonas sp. |
| 50 | Pseudomonas sp. |
| 51 | Proteus sp. |
| 52 | Streptococcus pneumoniae |
| 53 | Streptococcus pneumoniae |
| 54 | Streptococcus pneumoniae |
| 55 | Enterococcus faecium |
| 56 | Staphylococcus sp. |
| 57 | Staphylococcus sp. |
| 58 | Staphylococcus sp. |
This study evaluated the use of 16S rRNA gene PCR-hybridization as an aid in the diagnosis of bacteremia. The results showed that PCR-hybridization has strong concordance with culture, similar to previous reports (7, 11). The results also revealed that more than 10% of negative cultures converted to positive after PCR-hybridization testing and that PCR-hybridization detected nonvital bacteria after exposure to antibiotics.
For positive cultures, the one PCR-hybridization-negative sample was a CONS infection. The length of time to the culture positive result, sometimes indicative of contamination versus infection for CONS, was not available. For negative cultures, the majority (9 of 11) of the PCR-hybridization-positive results were identified as CONS. The clinical relevance of a CONS result is case specific, possibly indicating contamination in otherwise healthy patients or suggesting a potentially serious infection in patients with indwelling vascular access devices (e.g., catheters, central lines). The correlation of these results with the presence of attached devices was unable to be investigated in this study. The results suggest that the combination of PCR-hybridization and case-specific information may help identify clinically relevant bacteremia undetected by culture.
The results obtained for two serial cultures from one patient (before and after antibiotic therapy) suggest that PCR-hybridization is able to detect the presence of nonviable bacteria, which therefore cannot be grown in culture but which indicate the presence of a serious infection. This capability contributes to the superior sensitivity of PCR-hybridization compared to culture and has practical benefits in the clinical setting, such as for the patient who is on empirical antibiotic treatment before diagnosis.
Most previous studies (7, 11) focused on validation of culture-positive results by PCR methods with less attention to the conversion of negative cultures to positive results. The current study showed that 10% of negative cultures contained bacteria, highlighting the clinical need for a method able to detect those organisms that cannot be detected with culture methods. This study demonstrated that PCR-hybridization can complement culture methods by focusing on supplemental testing of cultures with negative results rather than confirmation of positive cultures. Such an approach would help maximize detection of clinically important bacteremia as early as possible.
In summary, performing PCR-hybridization from cultured blood samples allows analyses by both methods and provides results when conventional methods are unable to culture or identify the pathogen. The primary benefit of this approach is in supplemental analysis of culture-negative samples, and it has potential to provide improved sensitivity for diagnosis of serious blood infections and to detect pathogens that cannot be cultured.
Acknowledgments
We thank Mary-Kay Romeo for editing.
Footnotes
Published ahead of print on 23 March 2011.
REFERENCES
- 1. Bryan C. S. 1989. Clinical implications of positive blood cultures. Clin. Microbiol. Rev. 2:329–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edelmann L., et al. 2004. Cystic fibrosis carrier screening: validation of a novel method using BeadChip technology. Genet. Med. 6:431–438 [DOI] [PubMed] [Google Scholar]
- 3. Fontana C., Favaro M., Pelliccioni M., Pistoia E. S., Favalli C. 2005. Use of the MicroSeq 500 16S rRNA gene-based sequencing for identification of bacterial isolates that commercial automated systems failed to identify correctly. J. Clin. Microbiol. 43:615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorelenkov V., Antipov A., Lejnine S., Daraselia N., Yuryev A. 2001. Set of novel tools for PCR primer design. Biotechniques 31:1326–1330 [DOI] [PubMed] [Google Scholar]
- 5. Hall K. K., Lyman J. A. 2006. Updated review of blood culture contamination. Clin. Microbiol. Rev. 19:788–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hashmi G., et al. 2005. A flexible array format for large-scale, rapid blood group DNA typing. Transfusion 45:680–688 [DOI] [PubMed] [Google Scholar]
- 7. Jordan J. A., Durso M. B. 2005. Real-time polymerase chain reaction for detecting bacterial DNA directly from blood of neonates being evaluated for sepsis. J. Mol. Diagn. 7:575–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kocoglu M. E., Bayram A., Balci I. 2005. Evaluation of negative results of BacT/Alert 3D automated blood culture system. J. Microbiol. 43:257–259 [PubMed] [Google Scholar]
- 9. Kulski J. K., Pryce T. 1996. Preparation of mycobacterial DNA from blood culture fluids by simple alkali wash and heat lysis method for PCR detection. J. Clin. Microbiol. 34:1985–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li A. X., Seul M., Cicciarelli J., Yang J. C., Iwaki Y. 2004. Multiplexed analysis of polymorphisms in the HLA gene complex using bead array chips. Tissue Antigens 63:518–528 [DOI] [PubMed] [Google Scholar]
- 11. Liu Y., Han J. X., Huang H. Y., Zhu B. 2005. Development and evaluation of 16S rDNA microarray for detecting bacterial pathogens in cerebrospinal fluid. Exp. Biol. Med. (Maywood) 230:587–591 [DOI] [PubMed] [Google Scholar]
- 12. Lu G., Moriyama E. N. 2004. Vector NTI, a balanced all-in-one sequence analysis suite. Brief. Bioinform. 5:378–388 [DOI] [PubMed] [Google Scholar]
- 13. Millar B. C., Jiru X., Moore J. E., Earle J. A. 2000. A simple and sensitive method to extract bacterial, yeast and fungal DNA from blood culture material. J. Microbiol. Methods 42:139–147 [DOI] [PubMed] [Google Scholar]
- 14. Reimer L. G., Wilson M. L., Weinstein M. P. 1997. Update on detection of bacteremia and fungemia. Clin. Microbiol. Rev. 10:444–465 [DOI] [PMC free article] [PubMed] [Google Scholar]