Abstract
Vancomycin and daptomycin MICs from 161 isolates of methicillin-resistant Staphylococcus aureus (MRSA) were compared using commercial and in-house broth microdilution, Etest, and common automated methods. Vancomycin Etest MICs were higher than those of other methods, whereas the MICs for daptomycin testing were comparable. Vancomycin MICs vary depending on the testing methodology.
TEXT
Vancomycin is the gold standard for the treatment of serious infections due to methicillin-resistant Staphylococcus aureus (MRSA). Recent guidelines from the Infectious Diseases Society of America (IDSA) state that patients infected with MRSA strains that have a vancomycin MIC of ≥2 μg/ml have a higher probability of treatment failure and recommend changing to alternate therapy (9). It is essential that clinical microbiology laboratories accurately determine vancomycin MICs to guide appropriate therapy.
Trends toward increasing vancomycin MICs have been noted over the past 5 to 10 years (13). Hypotheses for the increase in vancomycin MICs include “MIC creep,” clonal shift, and variation in vancomycin MICs demonstrated with various testing methods (3, 10, 14). It is critical for laboratorians to be aware of the normal distribution of vancomycin MICs observed by the testing system in use in their institution to properly assess the possibility of MIC creep or strains of MRSA with falsely elevated vancomycin MICs. A “falsely” elevated MIC could lead to an unnecessary change in treatment.
The purpose of this study was to compare vancomycin and daptomycin MICs obtained by a variety of testing methods. A total of 161 consecutive blood culture isolates of MRSA were included in this study. The isolates were from Memorial Hermann Hospital, a 700-bed, private, tertiary care, university-affiliated teaching hospital located within the Texas Medical Center in Houston, Texas, between August 2005 and May 2007. Diversilab sequencing according to the manufacturer's instructions yielded at least seven clones (data not shown), as found in other studies of MRSA isolates from our institution (15). Vancomycin MICs were determined by broth microdilution (BMD) trays prepared commercially and in-house, agar-based methods including Etest (bioMérieux, Durham, NC) and agar dilution, as well as automated BMD methods BD Phoenix, Vitek 2, and Microscan. Daptomycin MICs were determined by commercially prepared BMD trays, Etest, and Microscan. Etest inoculums were performed by hand plating. Agar and broth methods were performed according to CLSI standards using strain ATCC 29213 for quality control with each day of testing (2). All MIC determinations were performed in duplicate, and all commercial systems were used according to the manufacturer's instructions. Agar plates were prepared by serially diluting vancomycin hydrochloride hydrate (Sigma, St. Louis, MO) in Mueller-Hinton (BBL) agar at the following concentrations: 0.25, 0.5, 0.75,1, 1.5, and 2.0 μg/ml. Custom-made frozen broth microdilution trays with an expanded dilution range of vancomycin and daptomycin were purchased from Trek Diagnostic Systems (Cleveland, OH) and were considered the reference method for this study. The final concentrations of vancomycin were 0.125, 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 3.0, 4.0, and 6.0 μg/ml. The final concentrations for daptomycin were 0.125, 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, and 8.0 μg/ml. Essential agreement was calculated as previously described (1).
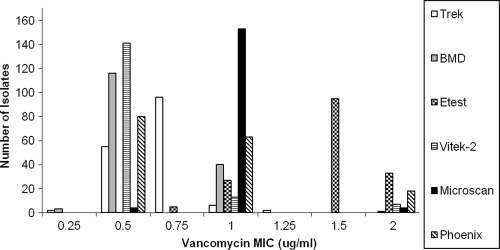
The results of vancomycin susceptibility testing by commercial and in-house broth microdilution, Etest, Vitek 2, Microscan, and Phoenix are shown in Fig. 1. All isolates were susceptible by all testing methods. The vancomycin MICs determined by the Etest method were consistently elevated than those determined by the reference commercially prepared frozen broth microdilution panels prepared by Trek. The vancomycin MIC mode was 0.75 μg/ml (96/161 [59.6%]) when measured by frozen Trek panels, compared to the Etest MIC mode of 1.5 μg/ml (95/161 [59.0%]). Using frozen Trek panels as the reference method, the essential agreement for in-house broth microdilution was 99.4%, while it was 76.4% for the Etest method, 96.3% for both Vitek 2 and Microscan, and 88.8% for Phoenix. Isolates that showed >2 dilutions of vancomycin MIC variation from frozen Trek panels were also tested by agar dilution. Vancomycin agar dilution MIC values were intermediate (mode of 1 μg/ml) between those obtained by frozen Trek panels (mode of 0.75 μg/ml) and Etest (mode of 1.5 μg/ml).
Fig. 1.
Vancomycin MIC distribution. MICs are shown in micrograms per milliliter. All isolates were tested using the six methods listed. Results are shown as the number of isolates at each MIC level.
Daptomycin MICs tested by frozen Trek panels, Etest, and Microscan are shown in Table 1. The mode MIC value was 0.5 μg/ml (118/161 [73.3%]) for the frozen Trek panels, and it was also 0.5 μg/ml (123/161 [76.4%]) for Microscan. The mode for Etest was 0.38 μg/ml (57/161 [35.4%]). The MICs obtained by frozen Trek panels were comparable to those of the Etest method, with an essential agreement of 97.5%. The essential agreement between frozen Trek panels and Microscan was 97.5%.
Table 1.
Daptomycin MIC distributiona
| Daptomycin MIC (μg/ml) | No. of isolates at each MIC level (% of total) determined by: |
||
|---|---|---|---|
| Trek | Etest | Microscan | |
| ≤0.25 | 5 (3.11) | 44 (27.33) | 37 (22.98)b |
| 0.38 | 57 (35.40) | ||
| 0.5 | 118 (73.29) | 32 (19.88) | 123 (76.40) |
| 0.75 | 35 (21.74) | 27 (16.77) | |
| 1 | 3 (1.86) | 1 (0.62) | 1 (0.62) |
| Essential agreement (%)c | 97.52 | 97.52 | |
All isolates were tested using the three available daptomycin MIC testing methods at the time of the study.
Data for Microscan are ≤0.25 μg/ml.
Essential agreement is obtained by comparison to custom-made frozen microdilution trays prepared by Trek.
Studies have demonstrated variability in vancomycin MICs among commercially available testing methods (3, 6, 8, 11, 14). Our results agree with other studies in that Etest showed an elevated MIC compared to BMD. Essential agreement for vancomycin MICs among our systems was 96% or higher with the exception of Etest, which showed an essential agreement of 76.4%. Swenson et al. found that Microscan, Etest, and Phoenix tended to generate results with elevated MICs compared to the broth reference method and agar dilution (14). However, Sensititre Vitek Legacy and Vitek 2 MICs tended to be lower than those of the broth reference method. Essential agreement of 98 to 100% was seen among all testing systems except for Vitek Legacy, which had an essential agreement of 90.6% (14). Similarly, two additional investigations found that vancomycin Etest MICs were consistently one 2-fold dilution higher than MICs generated by broth and agar dilution (6, 8). In a much larger study with 1,800 patients, both vancomycin and daptomycin MICs were higher when the MICs were determined by Etest than by broth microdilution (11). In comparison, the daptomycin MICs among the methods tested in this study were similar, with all having an essential agreement of 97.5% compared to reference broth.
In summary, the results of our study demonstrate that vancomycin MICs vary depending on the testing methodology. All of the isolates in this study were susceptible to vancomycin; we were thus unable to evaluate the accuracy of MIC determination of isolates with higher vancomycin MICs that are associated with treatment failure. To our knowledge, this is the first study to compare the vancomycin and daptomycin MICs from a variety of methods including automated systems as well as conventional broth- or agar-based methods on a large number of isolates. Studies have found that higher vancomycin MICs that are considered susceptible, such as MICs of ≥1.5 μg/ml, are associated with clinical failure (4, 5, 7, 12). The results of this study create a dilemma in that a majority of isolates had Etest vancomycin MICs of ≥1.5 μg/ml but were lower by other testing methods. It is essential that clinicians take the MIC testing method into consideration when choosing antimicrobial chemotherapy.
Acknowledgments
This work was supported in part by a grant from Cubist Pharmaceuticals Inc.
Footnotes
Published ahead of print on 30 March 2011.
REFERENCES
- 1. Baker C. N., Tenover F. C. 1996. Evaluation of Alamar colorimetric broth microdilution susceptibility testing method for staphylococci and enterococci. J. Clin. Microbiol. 34:2654–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. CLSI 2006. Performance standards for antimicrobial susceptibility testing. CLSI approved standard M100-S16. CLSI, Wayne, PA [Google Scholar]
- 3. Hsu D. I., et al. 2008. Comparison of method-specific vancomycin minimum inhibitory concentration values and their predictability for treatment outcome of meticillin-resistant Staphylococcus aureus (MRSA) infections. Int. J. Antimicrob. Agents 32:378–385 [DOI] [PubMed] [Google Scholar]
- 4. Lodise T. P., et al. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 52:3315–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maclayton D. O., Suda K. J., Coval K. A., York C. B., Garey K. W. 2006. Case-control study of the relationship between MRSA bacteremia with a vancomycin MIC of 2 μg/mL and risk factors, costs, and outcomes in inpatients undergoing hemodialysis. Clin. Ther. 28:1208–1216 [DOI] [PubMed] [Google Scholar]
- 6. Mason E. O., et al. 2009. Vancomycin MICs for Staphylococcus aureus vary by detection method and have subtly increased in a pediatric population since 2005. J. Clin. Microbiol. 47:1628–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moise P. A., Sakoulas G., Forrest A., Schentag J. J. 2007. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 51:2582–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prakash V., Lewis. J. S., II, Jorgensen J. H. 2008. Vancomycin MICs for methicillin-resistant Staphylococcus aureus isolates differ based upon the susceptibility test method used. Antimicrob. Agents Chemother. 52:4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rybak M. J., et al. 2009. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 49:325–327 [DOI] [PubMed] [Google Scholar]
- 10. Sader H. S., et al. 2009. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob. Agents Chemother. 53:4127–4132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sader H. S., Rhomberg P. R., Jones R. N. 2009. Nine-hospital study comparing broth microdilution and Etest method results for vancomycin and daptomycin against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:3162–3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soriano A., et al. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46:193–200 [DOI] [PubMed] [Google Scholar]
- 13. Steinkraus G., White R., Friedrich L. 2007. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J. Antimicrob. Chemother. 60:788–794 [DOI] [PubMed] [Google Scholar]
- 14. Swenson J. M., et al. 2009. Accuracy of commercial and reference susceptibility testing methods for detecting vancomycin-intermediate Staphylococcus aureus. J. Clin. Microbiol. 47:2013–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Welsh K. J., et al. 2010. Clinical characteristics, outcomes, and microbiologic features associated with methicillin-resistant Staphylococcus aureus bacteremia in pediatric patients treated with vancomycin. J. Clin. Microbiol. 48:894–899 [DOI] [PMC free article] [PubMed] [Google Scholar]