Fig.1.
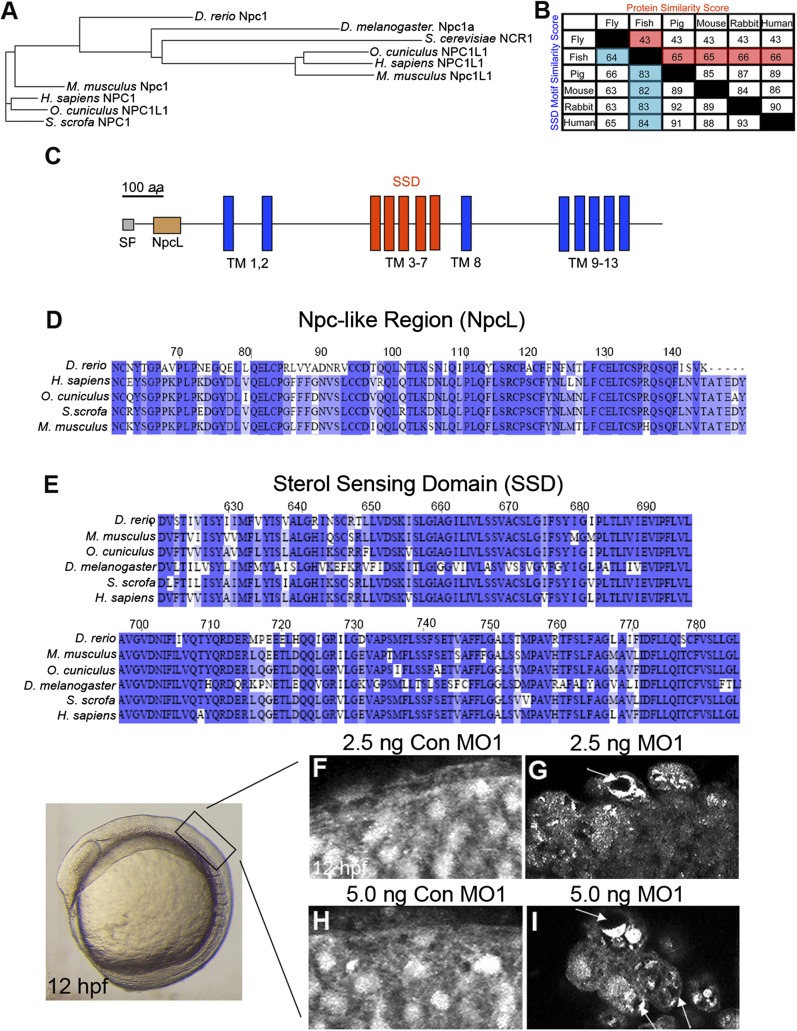
Zebrafish Npc1 protein and function is highly conserved. (A) Phylogenetic tree of D. rerio (zebrafish), D. melanogaster (fly), S. cerevisiae (yeast), H. sapiens (human), M. musculus (mouse), and S. scrofa (pig) NPC1 proteins, and O. cuniculus (rabbit), human, and mouse NPC1-like1 (Npc1L1) proteins. (B) Similarity score (the percentage of identical amino acids) for whole protein and the SSD motif among Npc1 amino acid sequences for a variety of species. Zebrafish column and row are highlighted. Common names are used for space considerations. (C) Zebrafish Npc1 protein contains a putative signal peptide (SP) at the N-terminus, 13 transmembrane (TM) spanning domains, a Npc-like region (NpcL), and a sterol sensing domain (SSD) motif. A line corresponding to the length of 100 amino acids is provided for reference. (D–E) Protein sequence alignments for the NpcL (D) and the SSD (E) are compared between zebrafish Npc1 and known NPC1 orthologs. Identical residues are shaded. The numbers above the aligned sequences in D and E correspond to the amino acid sequence of zebrafish Npc1. (F–I) Sterol localization defects in npc1 morphants, images taken from the region of a 12 hpf embryo detailed on the left. Lateral views, anterior to the left, of confocal stack projections obtained by imaging flat-mounted trunk tissue from 12 hpf embryos injected with 2.5-5.0 ng Con MO1 (F, H) or their stage-matched siblings injected with 2.5-5.0 ng MO1 (G, I). (F, H) In controls, filipin staining was diffuse and consistent throughout the field of cells. (G, I) In npc1 morphants, filipin staining appeared punctuate and uneven in many cells, often revealing darker areas within a single cell where the fluorescent signal was not equally dispersed (arrows).