Abstract
Purpose
To critically review perioperative outcomes, positive surgical margin (PSM) rates, and functional outcomes of several large series of retropubic radical prostatectomy (RRP), laparoscopic RP (LRP), and robot-assisted radical prostatectomy (RARP) currently available in the literature.
Methods
A Medline database search was performed from November 1994 to May 2009, using medical subject heading search terms “prostatectomy” and “Outcome Assessment (Health Care)” and text words “retropubic,” “robotic,” and “laparoscopic.” Only studies with a sample size of 250 or more patients were considered. Weighted means were calculated for all outcomes using the number of patients included in each study as the weighing factor.
Results
We identified 30 articles for RRP, 14 for LRP, and 14 for RARP. The mean intraoperative and postoperative RRP transfusion rates for RRP, LRP, and RARP were 20.1%, 3.5%, and 1.4%, respectively. The weighted mean postoperative complication rates for RRP, LRP, and RARP were 10.3% (4.8% to 26.9%), 10.98% (8.9 to 27.7%), and 10.3% (4.3% to 15.7%), respectively. RARP revealed a mean overall PSM rate of 13.6%, whereas LRP and RRP yielded a PSM of 21.3% and 24%, respectively. The weighted mean continence rates at 12 month follow-up for RRP, LRP, and RARP were 79%, 84.8%, and 92%, respectively. The weighted mean potency rates for patients who underwent unilateral or bilateral nerve sparing, at 12 month follow-up, were 43.1% and 60.6% for RRP, 31.1% and 54% for LRP, and 59.9% and 93.5% for RARP.
Conclusion
RRP, LRP, and RARP performed in high-volume centers are safe options for treatment of patients with localized prostate cancer, presenting similar overall complication rates. LRP and RARP, however, are associated with decreased operative blood loss and decreased risk of transfusion when compared with RRP. Our analysis including high-volume centers also showed lower weighted mean PSM rates and higher continence and potency rates after RARP compared with RRP and LRP. However, the lack of randomized trials precludes definitive conclusions.
Introduction
Prostate cancer is the most commonly diagnosed nonskin cancer and the second leading cause of cancer-related death in men in the United States.1,2 The incidence of prostate cancer rose dramatically in the late 1980s, reflecting improvements in detection through the widespread use of prostate-specific antigen (PSA) testing.2 As a result, prostate cancer is now a frequent diagnosis in younger and healthier men, with organ-confined disease, who desire to undergo definitive treatment, while maintaining their current quality of life. Because a myriad of treatment options is currently available, however, decision making for the patient can be confusing and stressful. Even among surgical options, the patient needs to decide whether to have open, laparoscopic or robot-assisted surgery.
Since Walsh and Donker3 first introduced the anatomic nerve-sparing technique for retropubic radical prostatectomy (RRP), it has become the gold standard and most widespread treatment for patients with clinically localized prostate cancer, providing excellent cancer control in most patients with clinically localized disease.4–6 In an effort to further decrease the morbidity of RRP, a minimally invasive surgical approach to managing prostate cancer was first described by Schuessler and colleagues7 in 1992. The initial experience with laparoscopic radical prostatectomy (LRP), however, was discouraging, and the authors concluded that the procedure was extremely difficult, associated with a steep learning curve, and offered no advantages over RRP. Subsequently, larger LRP series were reported showing feasibility of the procedure and results comparable to those of the open surgical approach.8–10 Despite this, the technical demands of the surgery and the protracted learning curve have prevented the widespread adoption of LRP by most urologic surgeons.
The introduction of the da Vinci Robotic Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA) has been a key step toward a minimally invasive approach to radical prostatectomy (RP) because of its technologic peculiarities, such as three-dimensional vision, seven degrees of freedom, and magnification. Almost one decade after the introduction of robot-assisted radical prostatectomy (RARP)11,12 and several modifications to the original surgical technique, the procedure is currently standardized. More importantly, the data from large series are now mature enough to be critically compared with other large RRP and LRP series.
The aim of our study was to critically review perioperative outcomes, positive surgical margin (PSM) rates, and functional outcomes of the largest RRP, LRP, and RARP series currently available in the literature.
Method
A Medline database search was performed from November 1994 to May 2009, using medical subject heading search terms “prostatectomy” and “Outcome Assessment (Health Care)” and text words “retropubic,” “robotic,” and “laparoscopic.” Additional hand searches based on references from relevant review articles were also performed.13–15 Only studies that were published in the English language and with a sample size of 250 or more patients were considered. Studies with a total sample size larger than 250 patients but with fewer patients specifically evaluated for continence or potency rates were also included in the analysis. Comparative studies were also included in the analysis and data from each group were pooled according to the surgical approach. No data from abstracts or reports from meetings were included in the review.
End points evaluated included perioperative outcomes (operative time, blood loss, transfusion rate, hospital stay, catheter time, and overall complication rates); functional outcomes (urinary continence, potency recovery); and PSM rates. Weighted means were calculated for all outcomes using the number of patients included in each study as the weighting factor.
Results
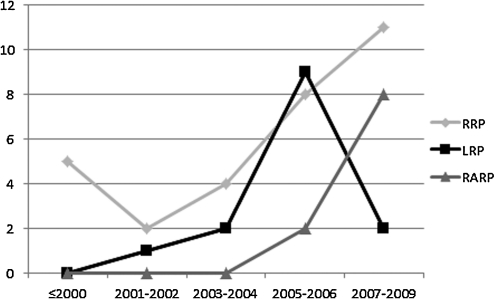
We identified 30 articles for RP, 14 for LRP, and 14 for RARP. The distribution of published articles over the years is shown in Figure 1. The weighted mean patient age for RRP, LRP, and RARP series included in the studies was 61.3 years (range of means, 58–65 years), 62.9 years (range of means, 57.6–64 years) and 60.4 years (range of means, 59.2–63.5 years), respectively.
FIG. 1.
Distribution of articles published by high-volume centers (>250 patients).
Perioperative outcomes
The results for operative time, blood loss, blood transfusion rates, hospital stay, and overall complication rates for RARP, LRP, and RRP are presented on Table 1,16–36, Table 2,33,37–47 and Table 3,28,30,31,43,48–54 respectively.
Table 1.
Open Radical Prostatectomy: Perioperative Outcomes
| |
|
|
|
|
|
Clinical stage |
|
|
|
|
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year of publication | Patients (N) | Surgical volume | Mean/median age (years) | Preop PSA (ng/mL) | cT1c | cT2 | Operative time (min) | EBL (mL) | % Transfused | Hospital length of stay (days) | Complication rate % |
| Zincke16 | 1994 | 1143 | 55 RRPs/y | 64 | - | 7 | 93 | - | - | 21.7 | 6 | - |
| Catalona17 | 1999 | 1870 | 133 RRPs/y | 63 | - | 30 | 62 | - | - | - | - | 10.5 |
| Grossfeld18 | 2000 | 1383 | 769 RRPs/ya | 63 | - | - | - | - | 818 | - | - | 6.6 |
| Lepor19 | 2001 | 1000 | 167 RRP/y | 60.3 | 7.6 | 77.7 | 21.3 | 131 | 813 | 8.2 | 2.3 | 6.5 |
| Hull20 | 2002 | 1000 | 67 RRPs/y | 62.9 | 6.8 | 33.2 | 57.8 | - | - | - | - | - |
| Augustin21 | 2003 | 1243 | 414 RRPs/y | 62.1 | 9.3 | 64.6 | 33.9 | - | 1284 | 29.1 | - | 19.8 |
| Han22 | 2004 | 9035 | 476 RRPs/y | 58.5 | - | 52.7 | 43.5 | - | - | - | - | - |
| Kundu23 | 2004 | 3477 | 357 RRPs/y | 61 | - | 51 | 45 | - | - | - | - | 9.1 |
| Saranchuk24 | 2005 | 647 | b | 58 | 6.9 | 56.2 | 41.5 | - | - | - | - | - |
| Bianco25 | 2005 | 1746 | 187 RRPs/y | - | - | 33 | 58 | - | - | - | - | - |
| Orvieto26 | 2006 | 996 | 93 RRPs/y | 60.1 | - | 60.4 | 39.2 | - | - | - | - | - |
| Sacco27 | 2006 | 985 | 76 RRPs/y | 64.5 | - | 17.5 | 68 | - | 750 | - | - | - |
| Nelson28 | 2007 | 374 | 334 RPs/y+ | 59.9 | 8.4 | - | - | - | - | - | 1.23 | 15 |
| Sengupta29 | 2008 | 6496 | 722 RRPs/y | 65 | 7.0 | 32.5 | 45.5 | - | - | - | - | - |
| Schroeck30 | 2008 | 435 | 234 RPs/y+ | 60.3 | 5.3 | 72.4 | 24.7 | - | 800 | - | - | - |
| Chan31 | 2008 | 340 | 113 RRPs/y | 61.2 | 8.24 | 66.2 | 32.6 | 141 | 503 | 3.2 | 1.4 | - |
| Eastham32 | 2008 | 1577 | 415 RRPs/y | 58 | 6.4 | 65 | 34 | - | - | - | - | - |
| Touijer33 | 2008 | 818 | 273 RRPs/y | 59 | 5.3 | 65 | 35 | 188 | 1267 | 49. | 3.3 | 6.6 |
| Loeb34 | 2008 | 3458 | 186 RRPs/y | 61 | 7.1 | 56 | 41 | - | - | - | - | 7 |
| Krambeck35 | 2009 | 588 | c | 61 | 5 | 71.1 | 26.9 | 204 | - | 13.1 | - | 4.8 |
| Constantinides36 | 2009 | 995 | 142 RRPs/y | 63.2 | 5.73 | - | - | - | - | 5.3 | - | 26.9 |
| Weighted means | 61.3 | 7.00 | 47.1% | 45.7% | 165 | 951 | 20.1% | 3.48 | 10.3 | |||
Multi-institutional study (CaPSURE database).
Total number of RPs included in the comparative study per year. Included in Nelson28 and Schroeck studies30 (RRP and RARP combined).
b = time frame of the study not reported; c = matched comparison RARP vs RRP. Total number of RRPs not reported; Preop = preoperative; PSA = prostate-specific antigen; EBL = estimated blood loss; RRP = retropubic radical prostatectomy; RP = radical prostatectomy; RARP = robot-assisted radical prostatectomy.
Table 2.
Laparoscopic Radical Prostatectomy: Perioperative Outcomes
| |
|
|
|
|
|
Clinical stage |
|
|
|
|
|
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year of publication | Patients (N) | Surgical volume | Mean/median age (years) | Preop PSA (ng/mL) | T1c (%) | T2 (%) | Operative time (min) | EBL (mL) | % Transfused | Open conversion (%) | Hospital length of stay (days) | Complication rate (%) |
| Guillonneau37 | 2003 | 1000 | 240 LRPs/y | 63 | 10 | 66.5 | 33.1 | - | - | - | - | - | - |
| Rossweiler38 | 2004 | 850 | 193 LRPs/y | 64 | 11.8 | - | - | 228 | - | - | - | - | - |
| Gonzalgo39 | 2005 | 250 | 83 LRPs/y | 57.6 | 6.4 | 68.8 | 30.8 | - | 2.8 | 1.6 | 2.5 | 13.8 | |
| Rozet40 | 2005 | 600 | 300 LRPs/y | 62 | 7.4 | 61 | 36.7 | 173 | 380 | 1.2 | 0.16 | 6.3 | 11.2 |
| Stolzenburg41 | 2005 | 700 | 234 LRPs/y | 63.4 | 10.7 | 151 | 220 | 0.9 | 0 | - | 12.1 | ||
| Lein42 | 2006 | 1000 | 230 LRPs/y | 62 | 8.78 | - | - | 266 | - | 2.2 | 0 | 7 | 12.8 |
| Hu43 | 2006 | 358 | 297 RPs/ya | 63.7 | - | 72.90 | 24.1 | 246 | 200 | 2.2 | 0.8 | 4.2 | 27.7 |
| Curto44 | 2006 | 425 | 370 LRPs/y | 62 | 7.14 | 65 | 32.8 | 100 | 200 | - | 4.3 | ||
| Goeman45 | 2006 | 550 | 110 LRPs/y | 62.4 | 10.1 | 77.40 | 20.6 | 188 | 390 | 4.7 | 0.5 | 4.6 | 10.9 |
| Rassweiler46 | 2006 | 5824 | 74LRPs/y/centerb | 64 | 196.4 | 4.1 | 2.4 | 8.9 | |||||
| Touijer33 | 2008 | 612 | 210 LRPs/y | 60 | 5.3 | 70 | 30 | 199 | 315 | 3.00 | - | 2 | 15.5 |
| Pavlovich47 | 2008 | 528 | 122 LRPs/y | 57.6 | 6.0 | 68.9 | 30.9 | - | - | - | 0.8 | - | |
| Weighted means | 62.9 | 8.8 | 69.2 | 30.4 | 205 | 291.5 | 3.5 | 1.76 | 4.87 | 10.98 | |||
Total number of radical prostatectomies included in the comparative study per year (LRP and RARP combined).
Multi-institutional study. Number of LRPs per year per instituiton.
Preop = preoperative; PSA = prostate-specific antigen; EBL = estimated blood loss; LRP = laparoscopic radical prostatectomy; RP = radical prostatectomy; RARP = robot-assisted radical prostatectomy.
Table 3.
Robot-Assisted Radical Prostatectomy: Perioperative Outcomes
| |
|
|
|
|
|
Clinical stage |
|
|
|
|
|
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year of publication | Patients (N) | Surgical volume | Mean/median age (year) | Preop PSA (ng/mL) | T1c | T2 | Operative time (min) | Mean EBL (mL) | % Transfused | Open conversion (%) | Hospital length of stay (days) | Complication rate (%) |
| Hu43 | 2006 | 322 | 297 RPs/ya | 62.1 | - | 74.50 | 24.80 | 186 | 250 | 1.60 | 0 | - | 14.60 |
| Joseph48 | 2006 | 325 | 162 RARPs/y | 60 | 6.6 | 81.00 | 19.00 | 130 | 196 | 1.30 | 0.00 | - | 8.60 |
| Badani49 | 2007 | 2766 | 461 RARPs/y | 60.2 | 6.4 | 77.30 | 22.00 | 154 | 142 | 1.50 | 0.10 | 1.14 | 12.20 |
| Nelson28 | 2007 | 629 | 334 RPs/yb | 59.3 | 6.4 | - | - | - | - | - | - | 1.17 | 17 |
| Borin50 | 2007 | 400 | c | 61.2 | 6.6 | 68 | 26.5 | - | 103.5 | - | - | 1 | - |
| Zorn51 | 2007 | 744 | 233 RARPs/y | 59.6 | 6.6 | 74 | 26 | 234 | 222 | 1.20 | 1.20 | 1.2 | - |
| Schroeck30 | 2008 | 362 | 234 RPs/y | 59.2 | 5.4 | 83.10 | 16.90 | - | 150 | - | 1.60 | - | - |
| Chan31 | 2008 | 660 | 220RARPs/y | 60 | 6.8 | 75.30 | 24.70 | 207 | 140 | 0.80 | 0.90 | 1.3 | - |
| Patel52 | 2008 | 1500 | c | 61 | 6.1 | 78 | 20 | 105 | 111 | 0.50 | 0 | 1.1 | 4.30 |
| Krambeck35 | 2009 | 294 | 120 RARPs/y | 61 | 4.9 | 72.8 | 26.9 | 236 | - | 5.1 | - | - | 8 |
| Murphy53 | 2009 | 400 | 154 RARPs/y | 60.2 | 7 | 69.70 | 30.30 | 186 | - | 2.5 | 0.30 | 3.1 | 15.70 |
| Ham54 | 2009 | 321 | 137 RARPs/y | 63.5 | 29.7 | - | - | 219 | 402 | - | - | 5.3 | 5.3 |
| Weighted means | 60.4 | 7.23 | 76.2 | 22.8 | 162.6 | 164.2 | 1.4 | 0.34 | 1.43 | 10.3 | |||
Total number of RPs included in the comparative study per year (LRP and RARP combined).
Total number of RPs included in the comparative study per year (RRP and RARP combined).
c = time frame of the study not reported; Preop = preoperative; PSA = prostate-specific antigen; EBL = estimated blood loss; RP = radical prostatectomy; RARP = robot-assisted radical prostatectomy.
The weighted means for operative time were 165 minutes (range 131–204 min) for RRP, 162.6 minutes (130–236 min) for the RARP series, and 205 minutes (100–266 min) for the LRP series. The mean estimated blood loss (EBL) for RRP, LRP, and RARP was 951 mL, 291.5 mL, and 164.2 mL, respectively. The mean intraoperative and postoperative RRP transfusion rates for RRP, LRP, and RARP were 20.1%, 3.5%, and 1.4%, respectively.
In terms of hospital stay, RP series account for a weighted mean of 3.48 days; the mean hospital stay for LRP and RARP was 4.87 and 1.43 days, respectively.
The weighted mean postoperative complication rates for RRP, RLP, and RARP were 10.3% (range of means 4.8%–26.9%), 10.98% (range of means 8.9%–27.7%), and 10.3% (range of means 4.3%–15.7%), respectively.
Some RARP cases needed conversion to laparoscopy or RRP, and some of the RLP cases were converted to open surgery. The mean open conversion rate for RARP was 0.34% (range of means 0%–1.6 %) and for LRP was 1.76% (range of means 0%–2.4%).
Oncologic outcomes
The pathologic stage and PSM rates for open, RLP, and RARP series are summarized on Table 4,16–22,24–28,30,31,33,55–57 Table 5,33,37,38,40–42,44–47 and Table 6,30,31,35,48–54,58 respectively.
Table 4.
Open Radical Prostatectomy: Oncologic Outcomes
| |
|
|
Pathologic staging |
Positive surgical margins |
||||
|---|---|---|---|---|---|---|---|---|
| Authors | Year of publication | Patients (N) | pT2 (%) | pT3 (%) | pT4 (%) | pT2 (%) | pT3 (%) | Overall (%) |
| Zincke16 | 1994 | 1143 | 53.2 | 34.7 | - | - | - | 12 |
| Grossfeld18 | 2000 | 1383 | 74.5 | 25.5 (pT3 + pT4) | 26 | 67.3 | 34 | |
| Lepor19 | 2001 | 1000 | 76.6 | 21.6 | 1.5 | - | - | 19.9 |
| Hull20 | 2002 | 1000 | 59.5 | 33.3 | - | - | - | 12.8 |
| Augustin21 | 2003 | 1243 | 67 | 31.3 | 1.4 | - | 21.4 | |
| Ward55 | 2004 | 7268 | 69 | 31 | - | 28 | 58 (+EPE) | 38 |
| Han22 | 2004 | 9035 | 58.2 | 38.3 | - | 7.7 | 26.9 | 14.7 |
| Saranchuk24 | 2005 | 647 | 76.5 | 21.5 | 1.9 | - | - | 13 |
| Bianco25 | 2005 | 1746 | 66 | - | - | - | 12 | |
| Orvieto26 | 2006 | 996 | 69.7 | 30.1 | - | 1.7 | 24.9 | 8.8 |
| Porter56 | 2006 | 752 | 54.6 | 37.6 | - | - | - | 37.6 |
| Sacco27 | 2006 | 985 | 57.1 | - | - | - | - | 13.7 |
| Chun57 | 2006 | 4277 | 64.4 | - | - | - | - | 21.5 |
| Sengupta29 | 2008 | 6496 | 69.1 | 25.7 (pT3 + pT4) | - | - | 38.2 | |
| Schroeck30 | 2008 | 435 | 74.5 | 25.5 (pT3 + pT4) | - | - | 28 | |
| Chan31 | 2008 | 340 | 66.4 | 33.1 | 0.5 | 22.5 | 56.7 | 34 |
| Eastham32 | 2008 | 1577 | 71 | 28 EPE 6 SVI | - | - | - | 11 |
| Touijer33 | 2008 | 818 | - | 32 EPE6 SVI | - | - | - | 11 |
| Krambeck35 | 2009 | 588 | 88.6 | 10.1% (pT3 + pT4) | - | - | 17 | |
| Weighted means | 64.3 | 31.5 | 1.54 | 16.8 | 42 | 24 | ||
EPE = extraprostatic extension; SVI = seminal vesicle invasion.
Table 5.
Laparoscopic Radical Prostatectomy: Oncologic Outcomes
| |
|
|
Pathologic staging |
Positive surgical margins |
||||
|---|---|---|---|---|---|---|---|---|
| Authors | Year of publication | Patients (N) | pT2 (%) | pT3 (%) | pT4 (%) | pT2 (%) | pT3 (%) | Overall (%) |
| Guillonneau37 | 2003 | 1000 | 77.5 | 21.9 | - | 15.5 | 31.1 | 19.2 |
| Rossweiler38 | 2004 | 850 | 58.4 | 36.5 | 3.7 | 7.08 | 38.6 | 22 |
| Rozet40 | 2005 | 600 | 72 | 28 | - | 14.6 | 26.2 | 17.8 |
| Stolzenburg41 | 2005 | 700 | 55.4 | 43.9 | 0.6 | 10.8 | 31.2 | 19.7 |
| Lein42 | 2006 | 1000 | 70.2 | 29.4 | 0.4 | 15 | 54.6 | 27 |
| Curto43 | 2006 | 425 | 58.5 | 41.3 | - | 21.9 | 43.3 | 30.7 |
| Rassweiler44 | 2006 | 5824 | 60.7 | 36.7 | 1.9 | 10.6 | 39.4 | 21.2 |
| Goeman45 | 2006 | 550 | 55.8 | 38.7 | 5.4 | 17.9 | 44.8 | 31.3 |
| Pavlovich47 | 2008 | 528 | 81.5 | 17.5 | - | 8.2 | 39.9 | 13.7 |
| Touijer33 | 2008 | 612 | - | 26 EPE 4 SVI |
- | - | - | 11 |
| Weighted means | - | - | 64 | 32.6 | 3.6 | 12.4 | 39.2 | 21.3 |
EPE = extraprostatic extension; SVI = seminal vesicle invasion.
Table 6.
Robot-Assisted Radical Prostatectomy: Oncologic Outcomes
| |
|
|
Pathologic staging |
Positive surgical margins |
||||
|---|---|---|---|---|---|---|---|---|
| Authors | Year of Publication | Patients (N) | pT2 (%) | pT3 (%) | pT4 (%) | pT2 (%) | pT3 (%) | Overall (%) |
| Joseph48 | 2006 | 325 | 81.00 | 19.00 | - | - | - | 13.00 |
| Badani49 | 2007 | 2766 | 77.70 | 22.00 | 0.30 | 13.00 | 35.00 | 12.30 |
| Zorn51 | 2007 | 744 | - | - | - | 12.90 | 44.80 | 18.80 |
| Borin52 | 2007 | 400 | 73.5 | 26.5 (pT3 + pT4) | 6.1 | 31.9 | 12.5 | |
| Tewari58 | 2008 | 700 | 83.5 | 13.6 | 2.9 | 5.4 | - | - |
| Schroeck30 | 2008 | 362 | 79.30 | 20.70 | 0 | - | - | 29.30 |
| Chan31 | 2008 | 660 | 80.60 | 19.40 | 0 | 11.30 | 45.00 | 17.90 |
| Patel52 | 2008 | 1500 | 78.30 | 19.50 | 1.50 | 4.00 | 34.00 | 9.30 |
| Murphy53 | 2009 | 400 | 70 | 29.80 | 0.20 | 9.60 | 42.30 | 19.20 |
| Krambeck35 | 2009 | 294 | 90.1 | 9.9 (pT3 + pT4) | - | - | 15.6 | |
| Ham54 | 2009 | 321 | 55.1 | 43.7 | 1.2 | - | - | 33.3 |
| Weighted means | 78.7 | 20.5 | 0.8 | 9.6 | 37.1 | 13.6 | ||
The pathologic stage in the RARP series was of 78.2% pT2 tumors and 20.5% pT3 tumors. LRPs were performed on 64% pT2 and 32.6% pT3 tumors, and RRPs on 64.3% pT2 and 31.5% pT3 tumors. RARP revealed a mean overall PSM rate of 13.6%, whereas LRP and RRP yielded a PSM of 21.3% and 24%, respectively. The mean PSM rate for pT2 and pT3 tumors in the RARP series was 9.6% and 37.1%, respectively; in the open series, it was 16.8% and 42%, respectively; and in the LRP series, it was 12.4% and 39.2%, respectively.
Urinary continence outcomes
Postoperative urinary continence outcomes for RARP, LRP, and RRP are tabulated in Table 7,16,17,23–25,27,32–35,59–61 Table 8,38,40–42,44–46,62 and Table 9.35,48,50,51,53,58,63,64
Table 7.
Open Radical Prostatectomy: Urinary Continence Outcomes
| |
|
|
|
|
% Urinary continence |
||||
|---|---|---|---|---|---|---|---|---|---|
| Authors | Year of publication | Patients (N) | Mean/median age (years) | Mean/median follow-up (months) | 1 month | 3 months | 6 months | 12 months | > 18 months |
| Zincke16 | 1994 | 1000 | 64 | 12 | - | - | - | 78.8 | - |
| Catalona17 | 1999 | 1325 | 63 | 18 | - | - | - | - | 92 |
| Kao59 | 2000 | 1069 | 63.6 | >6 | - | - | 67 | - | - |
| Stanford60 | 2000 | 1291 | 62.9 | >18 | - | - | 38.6 | 60.5 | 58.1 |
| Lepor61 | 2004 | 491 | 58.8 | 24 | - | 33.7 | 63.5 | 76.2 | 80 |
| Kundu23 | 2004 | 2737 | 61 | 65 | - | - | - | - | 93 |
| Saranchuk24 | 2005 | 647 | 58 | 15 | - | - | - | 87 | 93 |
| Bianco25 | 2005 | 1472 | 58 | 48 | - | - | - | 91 | 95 |
| Penson65 | 2005 | 1213 | 58% between 39–64 | 60 | - | - | 44 | 69 | 74 |
| Sacco27 | 2006 | 985 | 64.5 | 95.5 | - | 68 | 78 | 86.9 | 92.3 |
| Eastham32 | 2008 | 1577 | 58 | 60 | - | - | - | 79 | 94 |
| Touijer33 | 2008 | 222 | 59 | 23 | - | - | - | 75 | 82.8 |
| Loeb34 | 2008 | 3458 | 61 | >18 | - | - | - | - | 93 |
| Krambeck35 | 2009 | 496 | 61 | 12 | - | - | - | 93.7 | - |
| Weighted means | 61.1 | 57.5 | 55.6 | 80 | 88.2 | ||||
Table 8.
Laparoscopic Radical Prostatectomy: Urinary Continence Outcomes
| |
|
|
|
|
% Urinary continence |
||||
|---|---|---|---|---|---|---|---|---|---|
| Authors | Year of publication | Patients (N) | Mean/median age (years) | Follow-up (months) | 1 month | 3 months | 6 months | 12 months | >18 months |
| Guillonneau62 | 2002 | 255 | - | 12 | - | - | - | 82.3 | - |
| Rossweiler38 | 2004 | 500 | 64 | 24 | 28 | 51 | 70 | 84 | 97 |
| Rozet40 | 2005 | 600 | 62 | 12 | - | - | - | 84 | - |
| Stolzenburg41 | 2005 | 700 | 63.4 | 12 | - | 73.8 | 83.8 | 92 | - |
| Lein42 | 2006 | 952 | 62 | 28.8 | - | - | - | - | 76 |
| Rossweiler46 | 2006 | 5824 | 64 | 12 | - | - | - | 84.9 | - |
| Goeman 45 | 2006 | 550 | 62.4 | 12 | 38 | 61.9 | 77 | 82.9 | 90.9 |
| Curto44 | 2006 | 425 | 62 | 12 | - | 76 | 95 | 95 | - |
| Touijer33 | 2008 | 193 | 60 | 23 | - | - | - | 48 | 62.1 |
| Weighted means | - | - | 63.5 | - | 33.2 | 66 | 81.1 | 84.8 | 83.3 |
Table 9.
Robot-Assisted Radical Prostatectomy: Continence Outcomes
| |
|
|
|
|
% Urinary continence |
||||
|---|---|---|---|---|---|---|---|---|---|
| Authors | Year of Publication | Patients (N) | Median/mean age (years) | Follow-up (months) | Immediate | 1 month | 3 months | 6 months | 12 months |
| Joseph48 | 2006 | 325 | 60 | 6 | 24 | 56 | 93 | 96 | - |
| Menon63 | 2007 | 1142 | 60.2 | 12 | - | - | - | - | 92.00 |
| Borin50 | 2007 | 400 | 61.2 | 6 | - | 70.5 | 89 | 97 | - |
| Zorn51 | 2007 | 300 | 59.4 | 24 | - | 23.00 | 47.00 | 68.00 | 90.00 |
| Patel64 | 2007 | 500 | 63.2 | 12 | 27 | - | 89 | 95 | 97 |
| Tewari58 | 2008 | 214 (NR) | 64.3 | 13 | 13.1 | 35.2 | 50.2 | 61.9 | 82.1 |
| 304 (AR) | 62.8 | 13 | 27 | 59 | 76.6 | 85.6 | 91.2 | ||
| 182 (TR) | 61.2 | 6 | 38.4 | 82.5 | 91.3 | 97.1 | - | ||
| Murphy53 | 2009 | 395 | 60.2 | >18 | - | - | - | - | 91.40 |
| Krambeck35 | 2009 | 294 | 61 | 12 | - | - | - | - | 91.8 |
| Weighted means | 61.1 | 25.7 | 54.3 | 78.6 | 87.5 | 92 | |||
NR = no reconstruction; AR = anterior reconstruction; TR = total reconstruction.
Direct comparisons for urinary continence rates between different prostatectomy series are difficult because of variations in definitions, data collection methods, and length of follow-up. In this review, the definition of continence that was adopted to collect the data from the studies was the use of no absorbent pads or the use of one pad only for security. We identified 14 RRP, 9 LRP, and 8 RARP studies with more than 250 patients and a follow-up longer than 6 months. The RRP continence rates ranged from 33.7% to 68%, 38.6% to 78%, 60.5% to 93.7%, and 58.1 to 96% at 3, 6, 12, and 18 months of follow-up, respectively. The LRP continence rates ranged from 51% to 76%, 70% to 95%, 82.3% to 95%, and 62.1 to 97% at 3, 6, 12, and 18 months of follow-up, respectively. Reports for RARP revealed continence rates of 47% to 93%, 61.9% to 97%, and 82.1% to 97% at 3, 6, and 12 months of follow-up, respectively. The weighted mean continence rates at 12 months of follow-up for RRP, LRP, and RARP were 79%, 84.8%, and 92%, respectively.
Potency outcomes
The potency rates for RRP, LRP, and RARP are detailed on Table 1017,23,24,32–34,59,60,65–68 Table 11,33,38,40,41,44–46,62,69 and Table 12.35,48,51,53,63,64 As for continence, comparisons of the potency rates between different series are extremely difficult. Several ways to assess postoperative potency are used, including different questionnaires (International Index of Erectile Function, Expanded Prostate Cancer Index Composite) and distinct methods of data gathering (telephone, personal interviews, etc). The type of nerve-sparing procedure (unilateral, bilateral, nonnerve-sparing) and the use of medications can also influence the outcomes, in addition to the surgical approach adopted.
Table 10.
Open Radical Prostatectomy: Potency Outcomes
| |
|
|
|
Type of nerve sparing % |
|
Potency (%) |
Overall potency rates (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year of publication | Patients (N) | Mean/median age (years) | Unilateral NS | Bilateral NS | Follow-up (months) | Unilateral NS | Bilateral NS | 1 month | 3 months | 6 months | 12 months | > 18 months |
| Catalona17 | 1999 | 858 | 63 | 7 | 93 | 18 | 47 | 68 | - | - | - | - | 66.5 |
| Kao59 | 2000 | 1069 | 63.6 | - | - | - | - | - | - | - | 23 | - | - |
| Stanford60 | 2000 | 1042 | 62.9 | - | - | >18 | 41.4 | 44 | - | - | 7.9 | 14.7 | 18.5 |
| Kundu23 | 2004 | 1834 | 61 | 3.4 | 96.5 | >18 | 53 | 76 | - | - | - | - | 75 |
| Saranchuk24 | 2005 | 647 | 58 | 6.6 | 92.5 | 24 | - | 62 | - | - | - | 37 | 62 |
| Bianco25 | 2005 | 785 | 58 | 27.7 | 71.8 | 48 | - | - | - | - | - | - | 70 |
| Penson65 | 2005 | 1213 | 58% between 39–64 | - | - | 60 | 23 | 40 | - | - | 9 | 17 | 28 |
| Michl66 | 2006 | 411 | 62.2 | 29.7 | 70.3 | 12 | 29.8 | 54.5 | - | - | - | 47.6 | - |
| Eastham32 | 2008 | 1577 | 58 | 19 | 80 | 36 | - | - | - | - | - | 39 | 67 |
| Touijer33 | 2008 | 164 | 59 | 6 | 91 | 23 | - | - | - | - | - | - | 58.5 |
| Loeb34 | 2008 | 3458 | 61 | - | 92 | >18 | - | - | - | - | - | - | 70 |
| Ayyathurai67 | 2008 | 797 | 60.5 | 22 | 78 | 45 | 53 | 72 | - | - | - | - | 67.7 |
| Marien68 | 2009 | 634 | 57 | 12 | 87 | 24 | 47 | 61 | - | - | - | - | 59 |
| Weighted means | 60.6 | 13.8 | 87.6 | 43.1 | 60.6 | 29 | 61 | ||||||
NS = nerve sparing.
Table 11.
Laparoscopic Radical Prostatectomy: Sexual Potency Outcomes
| |
|
|
|
Type of nerve sparing (%) |
|
Potency (%) |
Overall potency rates (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year of publication | Patients (N) | Mean/median age (years) | Unilateral NS | Bilateral NS | Follow-up (months) | Unilateral NS | Bilateral NS | 1 months | 3 months | 6 months | 12 months | > 18 months |
| Guilonneau62 | 2002 | 47 (500) | - | - | 100 | 12 | - | 66 | - | - | - | 66a | - |
| Rossweiler38 | 2004 | 41 (850) | 64 | 11.7 | 10.1 | 12 | - | 67 | - | - | - | 67a | - |
| Rozet40 | 2005 | 600 | 62 | 21.30 | 63.6 | 12 | - | 43 | - | - | 43a | - | - |
| Stolzenburg41 | 2005 | 131 | 63.4 | 16.3 | 10.1 | 6 | 13.3 | 47.1 | - | 13 | 27.8 | - | - |
| Rossweiler46 | 2006 | 5824 | 64 | - | - | 12 | 31 | 52.5 | - | - | - | 52.5a | - |
| Goeman45 | 2006 | 550 | 62.4 | 20.7 | 67 | 12 | - | 64 | 22.2b | 28.7b | 47.4b | 78.6b | - |
| Rossweiler69 | 2006 | 357c | - | 44.3 | 55.7 | - | - | 67 | - | - | - | - | - |
| 205d | - | 29.3 | 70.7 | - | - | 76 | - | - | - | - | - | ||
| Curto44 | 2006 | 425 | 62 | - | 100 | 12 | - | - | - | 30 | 43 | 58.5 | - |
| Touijer33 | 2008 | 130 | 60 | 6 | 88 | 56.1 | |||||||
| Weighted means | - | - | 63.5 | 24.5 | 68.3 | - | 31.1 | 54 | - | 27.3 | 43.2 | 55.1 | - |
Bilateral nerve-sparing.
Age <60 years.
Retrograde nerve-sparing.
Antegrade nerve-sparing.
NS = nerve sparing.
Table 12.
Robot-Assisted Radical Prostatectomy: Potency Outcomes
| |
|
|
|
Type of nerve sparing (%) |
|
Potency (%) |
Overall potency rates (%) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year of publication | Patients (N) | Median/mean age (years) | Unilateral | Bilateral | Follow-up (months) | Unilateral NS | Bilateral NS | 3 months | 6 months | 12 months | > 18 months |
| Joseph48 | 2006 | 325 | 60 | 23.6 | 70 | 12 | 58 | 80.6 | - | 77.1 | - | - |
| Menon63 | 2007 | 1142 | 60.2 | 25.00a | 33.00b | >18 | - | 100 | - | - | 70b | 100b |
| Zorn51 | 2007 | 300 | 59.4 | 26.4 | 59.6 | 24 | 62 | 83 | 47 | 58 | 74 | 76.5 |
| Patel64 | 2007 | 500 | 63.2 | - | - | 12 | - | - | - | - | 78 | - |
| Krambeck35 | 2009 | 294 | 61 | 91 | 12 | - | - | - | - | 70 | - | |
| Murphy53 | 2009 | 395 | 60.2 | 28.2 of potent men |
65.3 of potent men |
12 | - | - | - | - | 62 | - |
| Weighted means | 60.7 | 59.9 | 93.5 | 47 | 58 | 70.7 | 95 | |||||
Unilateral veil.
Bilateral veil.
NS = nerve sparing.
We identified 13 RRP, 9 LRP, and 6 RARP studies with more than 250 patients and with reported potency rates beyond 1 year. Evaluating RRP, the weighted mean potency rates for patients who underwent unilateral or bilateral nerve sparing, at 12 months of follow-up, were 43.1% and 60.6%, respectively. The LRP weighted mean potency rates for patients who received unilateral and bilateral nerve-sparing procedures, at 12 months of follow-up, were 31.1% and 54%, respectively; Finally, RARP patients who received unilateral and bilateral nerve-sparing procedures had potency rates, at 12 months of follow-up, of 59.9% and 93.5%, respectively.
Discussion
There are some clear problems with comparing open RP vs LRP vs RARP for the treatment of patients with organ-confined prostate cancer: There are no randomized studies that compare the three surgical approaches; definitions used to describe positive margins, biochemical recurrence, urinary continence, and sexual function are not standardized and may differ significantly between series; finally, centers where RARP is performed are usually focused only in this approach, limiting their practice with open or laparoscopic procedures. Consequently, comparative randomized studies that evaluate the three approaches in the same institution are scarce and include small patient series, which represent the surgeons' learning curve.13,70
Recently, two systematic reviews and meta-analysis of the literature evaluated the results of LRP, RARP, and RRP. Parsons and Bennett14 conducted a meta-analysis of 19 observational studies that compared either laparoscopic or robotic-assisted with retropubic prostatectomy. Ficarra and associates13 subsequently evaluated the perioperative, functional, and oncologic results in 37 comparative studies (RRP to LRP, RRP to RARP, or LRP to RARP). Both reviews demonstrated significantly lower blood loss and transfusion rates for LRP and RARP series compared with open RRP. None of the studies was able to prove the advantage of any surgical approach in terms of functional and oncologic outcomes. However, as expected in a meta-analysis, these two studies evaluated case series or cohorts that directly compared the surgical approaches within the same study. Consequently, some of the largest noncomparative cohorts carried out by experienced surgeons were not included in the statistical analysis.
In our review, we evaluated only the largest series (more than 250 patients) published with the three surgical approaches. Our aim was to analyze the outcomes of high-volume centers, minimizing the potential bias of the initial learning curve for each procedure.
Perioperative outcomes
It is difficult to compare operative times between different series because of variations in reporting operative time (including or not setup and/or pelvic lymph node dissection). In our study, the weighted mean operative times for RRP and RARP were similar (165 and 162.6 min, respectively). The weighted mean operative time (205 min) was longer in those patients who were undergoing LRP compared with the other two approaches. Similarly, the systematic review conducted recently by Ficarra and colleagues13 suggested that RARP is more time consuming than RRP only in the earlier phase of the learning curve71 but that such differences disappeared with increased surgical experience. The same meta-analysis also showed that the operative time is significantly longer in those patients who are undergoing LRP compared with RRP.
Badani and colleagues49 recently reported their experience after 2766 RARPs. For the entire cohort, the mean surgical time (calculated from the time of Veress needle placement to skin closure) and the mean console time was 154 minutes and 116 minutes, respectively. They compared the results of their first 200 patients (group 1) with their last 200 patients (group 2). For group 1, the mean surgical and console times were 160 minutes and 121 minutes, respectively; for group 2, the mean surgical and console times were 131 minutes and 97 minutes, respectively. The robotic setup and docking time also decreased from 45 minutes in group 1 to 8 minutes in group 2.
Decreased intraoperative blood loss has been reported to be a hallmark advantage of laparoscopic/robot-assisted prostatectomy. Because most intraoperative blood loss originates from the venous sinuses, the tamponade effect created by pneumoperitoneum helps to diminish blood loss. In addition, early identification and precise ligation of vessels facilitate the limitation of blood loss. In our study, the weighted mean EBL and the mean intraoperative and postoperative RRP transfusion rates were also higher for RRP than LRP and RARP (mean EBL 951 mL vs 291.5 mL vs 164.2 mL, respectively; transfusion rates 23%, 3.5%, and 1.4%, respectively). Two recent meta-analyses of studies that directly compared RRP, LRP, and RARP confirmed that RARP is associated with decreased operative blood loss and decreased risk of transfusion compared with RRP.13,14 It is noteworthy to mention, however, that some transfusion rates reported in RRP series are significantly biased by autologous blood transfusion protocols, which have a clearly more liberal indication for transfusion during the procedure.33
The mean in-hospital stay was higher for LRP series than in RRP and RARP in our review (4.87, 3.48, and 1.43 days, respectively). These results, however, probably reflected the differences in the location where the series were carried out. Most of the LRP series were conducted in Europe, where patients often stay in the hospital until the urinary catheter is removed; in the United States, the patients are usually discharged quickly from the hospital after surgery because of cultural disparities and differences in the insurance reimbursement patterns.
Surgical complications after RP have been documented in various previous series but few have used standardized classification systems. The absence of consensus within the surgical community on the best way to report complications has hampered accurate comparisons between different series and techniques. Lepor and coworkers,19 for example, reported a 6.5% overall incidence of complications in a series of 1000 RRPs performed by a single surgeon. In their series, however, blood transfusion (9.7%) was not considered a complication. Most of the RARP series published include blood transfusion in their overall complication rates. Therefore, any direct comparison would be quite inaccurate.
Few studies that compare complications after large RRP, LRP, and RARP series are available in the literature. The results are conflicting. Hu and colleagues43 compared intraoperative and early postoperative complications of 358 consecutive LRPs with 322 RARPs and showed lower overall complication rates after RARP (27.7% vs 14.6%). Instead, Rozet and associates,40 in a matched-pair analysis of 133 extraperitoneal RARPs and 133 extraperitoneal pure LRP, demonstrated a higher overall complication rate after RARP (19.4% vs 9.1%, P = 0.01). Most available series that compare RRP and RARP have reported similar complication rates between these two approaches. Krambeck and coworkers35 recently showed comparable overall perioperative complication rates between RARP and RRP (8.0% vs 4.8%, P = 0.064). Similarly, Nelson and colleagues28 showed equivalent rates of unscheduled visits (RRP = 10%, RARP = 10%, P = 0.95) and readmissions (RRP = 5%, RARP = 7%, P = 0.12) because of postoperative complications between these two surgical approaches.
Ficarra and associates13 showed in a cumulative analysis of comparative reports that the overall complication rate is significantly higher in those patients who are undergoing RRP. The same report showed that the complication rates after RARP or LRP are similar. In our review, the weighted mean postoperative complication rates were similar between the three approaches (10.3%, 10.98%, and 10.3%, respectively).
Oncologic outcomes
PSM after RP is thought to be an independent predictive factor of biochemical recurrence, local recurrence, and development of distant metastasis.72 In the absence of long-term data that compare biochemical recurrence and disease-free survival between the different surgical approaches, data regarding PSM are most helpful as a surrogate for these outcomes. Few studies that compare PSM rates after RRP, LRP, and RARP are available, with conflicting results.
Parsons and Bennett14 evaluated the surgical margin status in 13 comparative studies and showed no significant differences in overall risk or incidence of PSM between RRP and LRP or RARP. Of note, in the subgroup analysis by tumor stage, there were no significant differences between the groups for T2 cancers or T3 cancers. Likewise, Schroek and colleagues,30 comparing 362 consecutive RARPs with 435 RRPs, found no significant difference in PSM rates between RARP and RRP. The risk of PSA recurrence was also not significantly different after adjusting for clinical (hazard ratio 0.82, 95% confidence interval [CI] 0.48–1.38; P = 0.448) and pathologic differences (0.94, 0.55–1.61; P = 0.824). Similarly, Krambeck and coworkers35 showed, in their matched-pair analysis, no difference in the PSM rates between RARP and RRP. The 3-year biochemical disease-free survival was also not significantly different.
Contrary to these results, Ficarra and associates,13 evaluating PSM rates in six studies that compared RARP and RRP, suggested a significant advantage in the population who underwent RARP (relative risk [RR]: 1.58; 95% CI of RR: 1.29–1.94; P < 0.00001). When analyzing the PSM rates in patients with organ-confined prostate cancer, RARP was associated with the lowest risk of PSMs (RR: 2.23; 95% CI of RR: 1.36–3.67; P = 0.002). The PSM rates observed after open RP and LRP were similar. Our analysis that included high-volume centers confirmed this trend to lower PSM rates after RARP compared with RRP and LRP, because this surgical approach revealed a weighted mean overall PSM rate of 13.6%, whereas LRP and RRP yielded a PSM rate of 21.3% and 24%, respectively. PSM rates for pT2 tumors were also lowest among RARP series (16.8% for RRP; 12.4% for LRP, and 9.6% for RARP). Randomized trials are necessary, however, before establishing definitive conclusions.
Urinary continence outcomes
Objective evaluation of continence outcomes after RP remains stalled by the lack of standardization among series. Most studies used no validated institutional questionnaires, and the outcomes were assessed by an open interview.
Very few data regarding continence are available in the studies that compared RARP with LRP or with RRP, and meaningful conclusions as to whether any particular approach delivers superior continence outcomes are impractical. Nevertheless, better continence rates were suggested after RARP in some previous studies. Tewari and coworkers73 showed, in a nonrandomized comparative study, that RARP provides earlier continence recovery compared with RRP. These authors reported a prospective comparison between 100 RRPs and 200 RARPs and demonstrated a shorter time to return of continence after RARP (median time 160 vs 44 days; P < 0.05).
Contrarily to these results, Krambeck and associates35 in a matched comparison of RRP and RARP, showed comparable continence rates at the 1-year follow-up (RARP 91.8%, RRP 93.7%, P = 0.344). Likewise Parsons and Bennett14 evaluated urinary continence at 1 year of follow-up in four comparative studies. There was no significant difference in urinary continence between LRP or RARP and RRP.
Ficarra and coworkers13 analyzed eight articles that provided data on urinary continence recovery after RRP or LRP. Considering the studies that evaluated the learning curve of LRP, the return of continence was better after RRP. The cumulative analysis suggested similar continence rates after RRP or LRP, however. The cumulative analysis that compared RARP with RRP was not possible because of the heterogeneity of the studies.13
In our review, the weighted mean continence rates at 12 months for RRP, LRP, and RARP were 80%, 84.8%, and 92%, respectively. Our data support the statement that the continence rates after RRP and LRP are similar. The RARP continence rates were higher in our study when compared with RRP and LRP. Randomized prospective studies are necessary, however, to accurately compare the continence rates between the three surgical approaches.
Potency outcomes
Potency is one of the most difficult outcomes to compare after RP. Factors other than the surgeon or the approach have a significant effect on recovery of potency, including patient age, type and quality of the nerve sparing, and use of medications. Also, the assessment of postoperative continence is not standardized, including nonvalidated questionnaires and open interviews.
Whether there is a difference in the potency rates after RRP, LRP, or RARP is still not clear. It has been proposed that RARP may prevent damage to the neurovascular bundle because the three-dimensional magnified vision offered by the Da Vinci Surgical System allows more precise dissection and prevents inadvertent incision, traction, or incorporation of the neurovascular bundle into the suture or clip. Tewari and colleagues73 demonstrated better and earlier potency recovery after RARP compared with RRP. Patients who underwent RARPs showed earlier return of erections (50% at a mean follow-up of 180 days vs 50% at a mean of 440 days after RRP) as well as a quicker return to intercourse (50% at 340 days vs 50% at 700 days for RRP) compared with RRP patients. Contrary to these results, the matched-pair analysis conducted by Krambeck and coworkers35 showed comparable potency rates between RARP and RRP at 1-year follow-up. (RARP 70.0%, RRP 62.8%, P = 0.081).
Regarding the comparison between RARP and LRP, Joseph and associates74 conducted a single retrospective study that compared noncontemporary series of patients who were evaluated for erectile function recovery after LRP and RARP. At 3 months after surgery, only a nonstatistically significant trend in favor of RARP was observed. In our review, the weighted mean potency rates for bilateral nerve sparing were higher for RARP compared with the other two surgical approaches (93% vs 60.6% for RRP vs 54% for LRP). The poor methodology of the published studies and the absence of randomized clinical trials prevent any definitive conclusions, however.
Our study has some limitations. Although we included only high-volume centers in our analysis, several centers have multiple surgeons who perform RARP and LRP. Therefore, the individual learning curve of each surgeon could have been a source of bias. In addition, the series analyzed in each group are not contemporary. The outcomes from the RRP series are based on less contemporary patient cohorts. Therefore, some of the patients in the open RRP series were operated on before widespread use of PSA screening; baseline patient characteristics were not consistently comparable—ie, the pathologic stage was more advanced in the RRP series compared with the RARP series. Finally, comparison of outcomes using weighted means has some inherent limitations; a single large series with suboptimal outcomes can determine a worse weighted mean even if excellent results are reported in several smaller series.
Conclusions
Our analysis of large RRP, LRP, and RARP series with a sample size of 250 or more patients has led to the following conclusions:
RRP, LRP, and RARP that are performed in high-volume centers are safe options for treatment of patients with localized prostate cancer, presenting similar overall complication rates.
LRP and RARP are associated with decreased operative blood loss and decreased risk of transfusion when compared with RRP.
Our analysis including high-volume centers showed lower weighted mean PSM rates and higher continence and potency rates after RARP compared with RRP and LRP. The lack of randomized trials and long-term follow-up studies that compare the three approaches, however, precludes definitive conclusions.
Abbreviations Used
- CI
confidence interval
- EBL
estimated blood loss
- LRP
laparoscopic radical prostatectomy
- PSA
prostate-specific antigen
- PSM
positive surgical margin
- RARP
robot-assisted radical prostatectomy
- RP
radical prostatectomy
- RR
relative risk
- RRP
retropubic radical prostatectomy
Disclosure Statement
No competing financial interests exist.
References
- 1.Meng MV. Elkin EP. Harlan SR, et al. Predictors of treatment after initial surveillance in men with prostate cancer: Results from CaPSURE. J Urol. 2003;170:2279–2283. doi: 10.1097/01.ju.0000094190.46523.b2. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF. Kosary CL. Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007. National Cancer Institute, Bethesda, Md. http://seer.cancer.gov/csr/1975_2007/ 2010. http://seer.cancer.gov/csr/1975_2007/ based on November 2009 SEER data submission, posted to the SEER web site.
- 3.Walsh PC. Donker PJ. Impotence following radical prostatectomy: Insight into etiology and prevention. J Urol. 1982;128:492–497. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 4.Bill-Axelson A. Holmberg L. Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352:1977–1984. doi: 10.1056/NEJMoa043739. [DOI] [PubMed] [Google Scholar]
- 5.Reiner WG. Walsh PC. An anatomical approach to the surgical management of the dorsal vein and Santorini's plexus during radical retropubic surgery. J Urol. 1979;121:198–200. doi: 10.1016/s0022-5347(17)56718-x. [DOI] [PubMed] [Google Scholar]
- 6.Oelrich TM. The urethral sphincter muscle in the male. Am J Anat. 1980;158:229–246. doi: 10.1002/aja.1001580211. [DOI] [PubMed] [Google Scholar]
- 7.Schuessler WW. Schulam PG. Clayman RV. Kavoussi LR. Laparoscopic radical prostatectomy: Initial short-term experience. Urology. 1997;50:854–857. doi: 10.1016/S0090-4295(97)00543-8. [DOI] [PubMed] [Google Scholar]
- 8.Guillonneau B. Vallancien G. Laparoscopic radical prostatectomy: The Montsouris experience. J Urol. 2000;163:418–422. doi: 10.1016/s0022-5347(05)67890-1. [DOI] [PubMed] [Google Scholar]
- 9.Rassweiler J. Sentker L. Seemann O, et al. Laparoscopic radical prostatectomy with the Heilbronn technique: An analysis of the first 180 cases. J Urol. 2001;166:2101–2108. [PubMed] [Google Scholar]
- 10.Eden CG. Cahill D. Vass JA, et al. Laparoscopic radical prostatectomy: The initial UK series. BJU Int. 2002;90:876–882. doi: 10.1046/j.1464-410x.2002.03049.x. [DOI] [PubMed] [Google Scholar]
- 11.Binder J. Kramer W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001;87:408–410. doi: 10.1046/j.1464-410x.2001.00115.x. [DOI] [PubMed] [Google Scholar]
- 12.Pasticier G. Rietbergen JB. Guillonneau B, et al. Robotically assisted laparoscopic radical prostatectomy: Feasibility study in men. Eur Urol. 2001;40:70–74. doi: 10.1159/000049751. [DOI] [PubMed] [Google Scholar]
- 13.Ficarra V. Novara G. Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: A systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–1063. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 14.Parsons JK. Bennett JL. Outcomes of retropubic, laparoscopic, and robotic-assisted prostatectomy. Urology. 2008;72:412–416. doi: 10.1016/j.urology.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 15.Berryhill R., Jr Jhaveri J. Yadav R, et al. Robotic prostatectomy: A review of outcomes compared with laparoscopic and open approaches. Urology. 2008;72:15–23. doi: 10.1016/j.urology.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 16.Zincke H. Bergstralh EJ. Blute ML, et al. Radical prostatectomy for clinically localized prostate cancer: Long-term results of 1,143 patients from a single institution. J Clin Oncol. 1994;12:2254–2263. doi: 10.1200/JCO.1994.12.11.2254. [DOI] [PubMed] [Google Scholar]
- 17.Catalona WJ. Carvalhal GF. Mager DE. Smith DS. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol. 1999;162:433–438. [PubMed] [Google Scholar]
- 18.Grossfeld GD. Chang JJ. Broering JM, et al. Impact of positive surgical margins on prostate cancer recurrence and the use of secondary cancer treatment: Data from the CaPSURE database. J Urol. 2000;163:1171–1177. [PubMed] [Google Scholar]
- 19.Lepor H. Nieder AM. Ferrandino MN. Intraoperative and postoperative complications of radical retropubic prostatectomy in a consecutive series of 1,000 cases. J Urol. 2001;166:1729–1733. [PubMed] [Google Scholar]
- 20.Hull GW. Rabbani F. Abbas F, et al. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167:528–534. doi: 10.1016/S0022-5347(01)69079-7. [DOI] [PubMed] [Google Scholar]
- 21.Augustin H. Hammerer P. Graefen M, et al. Intraoperative and perioperative morbidity of contemporary radical retropubic prostatectomy in a consecutive series of 1243 patients: Results of a single center between 1999 and 2002. Eur Urol. 2003;43:113–118. doi: 10.1016/s0302-2838(02)00495-5. [DOI] [PubMed] [Google Scholar]
- 22.Han M. Partin AW. Chan DY, et al. An evaluation of the decreasing incidence of positive surgical margins in a large retropubic prostatectomy series. J Urol. 2004;171:23–26. doi: 10.1097/01.ju.0000098604.09395.27. [DOI] [PubMed] [Google Scholar]
- 23.Kundu SD. Roehl KA. Eggener SE, et al. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol. 2004;172:2227–2231. doi: 10.1097/01.ju.0000145222.94455.73. [DOI] [PubMed] [Google Scholar]
- 24.Saranchuk JW. Kattan MW. Elkin E, et al. Achieving optimal outcomes after radical prostatectomy. J Clin Oncol. 2005;23:4146–4151. doi: 10.1200/JCO.2005.12.922. [DOI] [PubMed] [Google Scholar]
- 25.Bianco Jr FJ. Scardino PT. Eastham JA. Radical prostatectomy: Long-term cancer control and recovery of sexual and urinary function (‘‘trifecta’’) Urology. 2005;66(suppl 5):83–94. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 26.Orvieto MA. Alsikafi NF. Shalhav AL, et al. Impact of surgical margin status on long-term cancer control after radical prostatectomy. BJU Int. 2006;98:1199–1203. doi: 10.1111/j.1464-410X.2006.06563.x. [DOI] [PubMed] [Google Scholar]
- 27.Sacco E. Prayer-Galetti T. Pinto F, et al. Urinary incontinence after radical prostatectomy: Incidence by definition, risk factors and temporal trend in a large series with a long-term follow-up. BJU Int. 2006;97:1234–1241. doi: 10.1111/j.1464-410X.2006.06185.x. [DOI] [PubMed] [Google Scholar]
- 28.Nelson B. Kaufman M. Broughton G, et al. Comparison of length of hospital stay between radical retropubic prostatectomy and robotic assisted laparoscopic prostatectomy. J Urol. 2007;177:929–931. doi: 10.1016/j.juro.2006.10.070. [DOI] [PubMed] [Google Scholar]
- 29.Sengupta S. Blute ML. Bagniewski SM, et al. After radical retropubic prostatectomy ‘insignificant’ prostate cancer has a risk of progression similar to low-risk ‘significant’ cancer. BJU Int. 2008;101:170–174. doi: 10.1111/j.1464-410X.2007.07270.x. [DOI] [PubMed] [Google Scholar]
- 30.Schroeck FR. Sun L. Freedland SJ, et al. Comparison of prostate-specific antigen recurrence-free survival in a contemporary cohort of patients undergoing either radical retropubic or robot-assisted laparoscopic radical prostatectomy. BJU Int. 2008;102:28–32. doi: 10.1111/j.1464-410X.2008.07607.x. [DOI] [PubMed] [Google Scholar]
- 31.Chan RC. Barocas DA. Chang SS, et al. Effect of a large prostate gland on open and robotically assisted laparoscopic radical prostatectomy. BJU Int. 2008;101:1140–1144. doi: 10.1111/j.1464-410X.2007.07428.x. [DOI] [PubMed] [Google Scholar]
- 32.Eastham JA. Scardino PT. Kattan MW. Predicting an optimal outcome after radical prostatectomy: The trifecta nomogram. J Urol. 2008;179:2207–2211. doi: 10.1016/j.juro.2008.01.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Touijer K. Eastham JA. Secin FP, et al. Comprehensive prospective comparative analysis of outcomes between open and laparoscopic radical prostatectomy conducted in 2003 to 2005. J Urol. 2008;179:1811–1817. doi: 10.1016/j.juro.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loeb S. Roehl KA. Helfand BT. Catalona WJ. Complications of open radical retropubic prostatectomy in potential candidates for active monitoring. Urology. 2008;72:887–891. doi: 10.1016/j.urology.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 35.Krambeck AE. DiMarco DS. Rangel LJ, et al. Radical prostatectomy for prostatic adenocarcinoma: A matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009;103:448–453. doi: 10.1111/j.1464-410X.2008.08012.x. [DOI] [PubMed] [Google Scholar]
- 36.Constantinides CA. Tyritzis SI. Skolarikos A, et al. Short- and long-term complications of open radical prostatectomy according to the Clavien classification system. BJU Int. 2009;103:336–340. doi: 10.1111/j.1464-410X.2008.08080.x. [DOI] [PubMed] [Google Scholar]
- 37.Guillonneau B. el-Fettouh H. Baumert H, et al. Laparoscopic radical prostatectomy: oncological evaluation after 1,000 cases a Montsouris Institute. J Urol. 2003;169:1261–1266. doi: 10.1097/01.ju.0000055141.36916.be. [DOI] [PubMed] [Google Scholar]
- 38.Rassweiler J. Schulze M. Teber D, et al. Laparoscopic radical prostatectomy: Functional and oncological outcomes. Curr Opin Urol. 2004;14:75–82. doi: 10.1097/00042307-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalgo ML. Pavlovich CP. Trock BJ, et al. Classification and trends of perioperative morbidities following laparoscopic radical prostatectomy. J Urol. 2005;174:135–139. doi: 10.1097/01.ju.0000161607.04334.26. [DOI] [PubMed] [Google Scholar]
- 40.Rozet F. Galiano M. Cathelineau X, et al. Extraperitoneal laparoscopic radical prostatectomy: A prospective evaluation of 600 cases. J Urol. 2005;174:908–911. doi: 10.1097/01.ju.0000169260.42845.c9. [DOI] [PubMed] [Google Scholar]
- 41.Stolzenburg JU. Rabenalt R. DO M, et al. Endoscopic extraperitoneal radical prostatectomy: Oncological and functional results after 700 procedures. J Urol. 2005;174:1271–1275. doi: 10.1097/01.ju.0000173940.49015.4a. [DOI] [PubMed] [Google Scholar]
- 42.Lein M. Stibane I. Mansour R, et al. Complications, urinary continence, and oncologic outcome of 1000 laparoscopic transperitoneal radical prostatectomies—experience at the Charité Hospital Berlin, Campus Mitte. Eur Urol. 2006;50:1278–1284. doi: 10.1016/j.eururo.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 43.Hu JC. Nelson RA. Wilson TG, et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J Urol. 2006;175:541–546. doi: 10.1016/S0022-5347(05)00156-4. [DOI] [PubMed] [Google Scholar]
- 44.Curto F. Benijts J. Pansadoro A, et al. Nerve sparing laparoscopic radical prostatectomy: Our technique. Eur Urol. 2006;49:344–352. doi: 10.1016/j.eururo.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 45.Goeman L. Salomon L. La De Taille A, et al. Long-term functional and oncological results after retroperitoneal laparoscopic prostatectomy according to a prospective evaluation of 550 patients. World J Urol. 2006;24:281–288. doi: 10.1007/s00345-006-0054-6. [DOI] [PubMed] [Google Scholar]
- 46.Rassweiler J. Stolzenburg J. Sulser T, et al. Laparoscopic radical prostatectomy—the experience of the German Laparoscopic Working Group. Eur Urol. 2006;49:113–119. doi: 10.1016/j.eururo.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Pavlovich CP. Trock BJ. Sulman A, et al. 3-year actuarial biochemical recurrence-free survival following laparoscopic radical prostatectomy: experience from a tertiary referral center in the United States. J Urol. 2008;179:917–922. doi: 10.1016/j.juro.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 48.Joseph JV. Rosenbaum R. Madeb R, et al. Robotic extraperitoneal radical prostatectomy: An alternative approach. J Urol. 2006;175:945–951. doi: 10.1016/S0022-5347(05)00340-X. [DOI] [PubMed] [Google Scholar]
- 49.Badani KK. Kaul S. Menon M. Evolution of robotic radical prostatectomy: Assessment after 2766 procedures. Cancer. 2007;110:1951–1958. doi: 10.1002/cncr.23027. [DOI] [PubMed] [Google Scholar]
- 50.Borin JF. Skarecky DW. Narula N. Ahlering TE. Impact of urethral stump length on continence and positive surgical margins in robot-assisted laparoscopic prostatectomy. Urology. 2007;70:173–178. doi: 10.1016/j.urology.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 51.Zorn KC. Gofrit ON. Orvieto MA, et al. Robotic-assisted laparoscopic prostatectomy: functional and pathologic outcomes with interfascial nerve preservation. Eur Urol. 2007;51:755–763. doi: 10.1016/j.eururo.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 52.Patel VR. Palmer KJ. Coughlin G. Samavedi S. Robot-assisted laparoscopic radical prostatectomy: Perioperative outcomes of 1500 cases. J Endourol. 2008;22:2299–2305. doi: 10.1089/end.2008.9711. [DOI] [PubMed] [Google Scholar]
- 53.Murphy DG. Kerger M. Crowe H, et al. Operative details and oncological and functional outcome of robotic-assisted laparoscopic radical prostatectomy: 400 cases with a minimum of 12 months follow-up. Eur Urol. 2009;55:1358–1366. doi: 10.1016/j.eururo.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 54.Ham WS. Park SY. Rha KH, et al. Robotic radical prostatectomy for patients with locally advanced prostate cancer is feasible: Results of a single-institution study. J Laparoendosc Adv Surg Tech A. 2009;19:329–332. doi: 10.1089/lap.2008.0344. [DOI] [PubMed] [Google Scholar]
- 55.Ward JF. Zincke H. Bergstralh EJ, et al. The impact of surgical approach (nerve bundle preservation versus wide local excision) on surgical margins and biochemical recurrence following radical prostatectomy. J Urol. 2004;172:1328–1332. doi: 10.1097/01.ju.0000138681.64035.dc. [DOI] [PubMed] [Google Scholar]
- 56.Porter CR. Kodama K. Gibbons RP, et al. 25-year prostate cancer control and survival outcomes: A 40-year radical prostatectomy single institution series. J Urol. 2006;176:569–574. doi: 10.1016/j.juro.2006.03.094. [DOI] [PubMed] [Google Scholar]
- 57.Chun FK. Graefen M. Zacharias M, et al. Anatomic radical retropubic prostatectomy-long-term recurrence-free survival rates for localized prostate cancer. World J Urol. 2006;24:273–280. doi: 10.1007/s00345-006-0058-2. [DOI] [PubMed] [Google Scholar]
- 58.Tewari A. Jhaveri J. Rao S, et al. Total reconstruction of the vesico-urethral junction. BJU Int. 2008;101:871–877. doi: 10.1111/j.1464-410X.2008.07424.x. [DOI] [PubMed] [Google Scholar]
- 59.Kao TC. Cruess DF. Garner D, et al. Multicenter patient self-reporting questionnaire on impotence, incontinence and stricture after radical prostatectomy. J Urol. 2000;163:858–864. [PubMed] [Google Scholar]
- 60.Stanford JL. Feng Z. Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354–360. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 61.Lepor H. Kaci L. The impact of open radical retropubic prostatectomy on continence and lower urinary tract symptoms: A prospective assessment using validated self-administered outcome instruments. J Urol. 2004;171:1216–1219. doi: 10.1097/01.ju.0000113964.68020.a7. [DOI] [PubMed] [Google Scholar]
- 62.Guillonneau B. Cathelineau X. Doublet JD, et al. Laparoscopic radical prostatectomy: Assessment after 550 procedures. Crit Rev Oncol Hematol. 2002;43:123–133. doi: 10.1016/s1040-8428(02)00024-0. [DOI] [PubMed] [Google Scholar]
- 63.Menon M. Shrivastava A. Kaul S, et al. Vattikuti Institute prostatectomy: Contemporary technique and analysis of results. Eur Urol. 2007;51:648–657. doi: 10.1016/j.eururo.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 64.Patel VR. Thaly R. Shah K. Robotic radical prostatectomy: Outcomes of 500 cases. BJU Int. 2007;99:1109–1112. doi: 10.1111/j.1464-410X.2007.06762.x. [DOI] [PubMed] [Google Scholar]
- 65.Penson DF. McLerran D. Feng Z, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol. 2005;173:1701–1705. doi: 10.1097/01.ju.0000154637.38262.3a. [DOI] [PubMed] [Google Scholar]
- 66.Michl UH. Friedrich MG. Graefen M, et al. Prediction of postoperative sexual function after nerve sparing radical retropubic prostatectomy. J Urol. 2006;176:227–231. doi: 10.1016/S0022-5347(06)00632-X. [DOI] [PubMed] [Google Scholar]
- 67.Ayyathurai R. Manoharan M. Nieder AM, et al. Factors affecting erectile function after radical retropubic prostatectomy: Results from 1620 consecutive patients. BJU Int. 2008;101:833–836. doi: 10.1111/j.1464-410X.2007.07409.x. [DOI] [PubMed] [Google Scholar]
- 68.Marien T. Sankin A. Lepor H. Factors predicting preservation of erectile function in men undergoing open radical retropubic prostatectomy. J Urol. 2009;181:1817–1822. doi: 10.1016/j.juro.2008.11.105. [DOI] [PubMed] [Google Scholar]
- 69.Rassweiler J. Wagner AA. Moazin M, et al. Anatomic nerve-sparing laparoscopic radical prostatectomy: Comparison of retrograde and antegrade techniques. Urology. 2006;68:587–592. doi: 10.1016/j.urology.2006.03.082. [DOI] [PubMed] [Google Scholar]
- 70.Nelson JB. Debate: Open radical prostatectomy vs. laparoscopic vs. robotic. Urol Oncol. 2007;25:490–493. doi: 10.1016/j.urolonc.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 71.Fracalanza S. Ficarra V. Cavalleri S, et al. Is robotically assisted laparoscopic radical prostatectomy less invasive than retropubic radical prostatectomy? Results from a prospective, unrandomized, comparative study. BJU Int. 2008;101:1145–1149. doi: 10.1111/j.1464-410X.2008.07513.x. [DOI] [PubMed] [Google Scholar]
- 72.Pfitzenmaier J. Pahernik S. Tremmel T, et al. Positive surgical margins after radical prostatectomy: Do they have an impact on biochemical or clinical progression? BJU Int. 2008;102:1413–1418. doi: 10.1111/j.1464-410X.2008.07791.x. [DOI] [PubMed] [Google Scholar]
- 73.Tewari A. Srivasatava A. Menon M Members of the VIP Team. A prospective comparison of radical retropubic and robot-assisted prostatectomy: Experience in one institution. BJU Int. 2003;92:205–210. doi: 10.1046/j.1464-410x.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 74.Joseph JV. Vicente I. Madeb R, et al. Robot-assisted vs pure laparoscopic radical prostatectomy: Are there any differences? BJU Int. 2005;96:39–42. doi: 10.1111/j.1464-410X.2005.05563.x. [DOI] [PubMed] [Google Scholar]