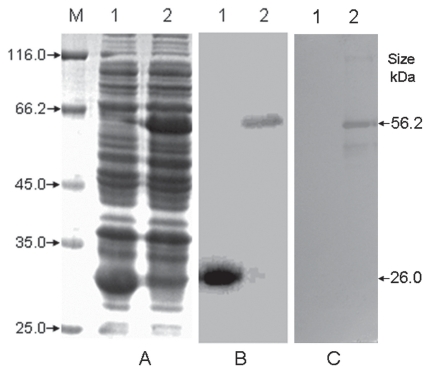
Figure 1.
Expression (A), purification (B), and Western blot analysis (C) of the recombinant TonB2 fusion protein. Protein samples were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis, blotted onto nitrocellulose membranes, and stained with Coomassie brilliant blue. The membranes were incubated with the primary antibody (porcine antibody against Actinobacillus pleuropneumoniae), to which was added horseradish peroxidase-conjugated goat IgG against porcine antigen. A 3,3′-diaminobenzidine kit was used for color development. M — protein marker with low molecular weight (Fermentas, Vilnius, Lithuania); 1 — glutathione-S-transferase (GST); 2 — GST-TonB2. The recombinant TonB2 protein exhibited a band with a molecular weight of approximately 56 kD, whereas no signal was detectable for the recombinant GST control.