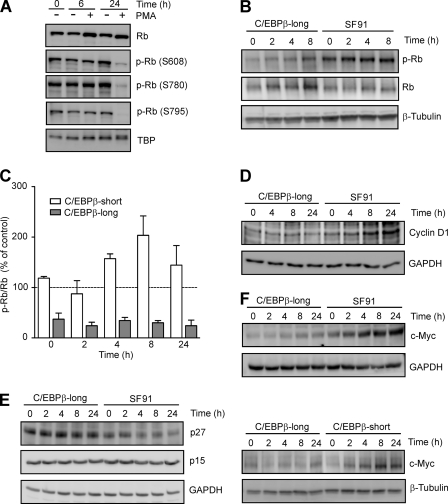
FIGURE 8.
Decreased Rb phosphorylation in PMA-treated THP-1 as well as C/EBPβ-long cells accompanied by decreased cyclin D1, increased p27, and decreased c-Myc levels. A, detection of hypophosphorylated Rb in THP-1 cells following exposure to PMA. THP-1 cells were incubated with 100 nm PMA up to 24 h. Nuclear total Rb protein and phosphorylated Rb protein (p-Rb; Ser608, Ser780, and Ser795) were detected by Western blot analysis. TBP was used as loading control. B, detection of hypophosphorylated Rb in C/EBPβ-long cells. C/EBPβ-long and SF91 cells were synchronized by serum starvation for 90 h and subsequently incubated in the presence of serum up to the indicated time points. Phospho-Rb (Ser780) and total Rb protein were monitored by Western blot analysis in whole cell extracts (loading control, β-tubulin). C, lower level of Rb phosphorylation in C/EBPβ-long compared with C/EBPβ-short cells. C/EBPβ-long, C/EBPβ-short, and SF91 cells were treated as described in B. The degree of Rb phosphorylation (Ser780) was calculated by dividing the signal for phospho-Rb (measured by densitometry) by that for total Rb protein. The phospho-Rb/Rb values in C/EBPβ-long and C/EBPβ-short cells were then compared with the respective values in SF91 control cells representing the 100% value at each time point (dashed line). Shown is the analysis of two experiments (mean ± S.D. (error bars)), which are representative of 10 independent experiments. D–F, decreased cyclin D1, increased p27, and reduced c-Myc levels in C/EBPβ-long cells. C/EBPβ-long, SF91, and C/EBPβ-short cells were cultured as described in B. Subsequently, protein levels of cyclin D1 (D), p27 and p15 (E), and c-Myc (F) were determined in whole cell extracts (loading control, GAPDH, or β-tubulin).