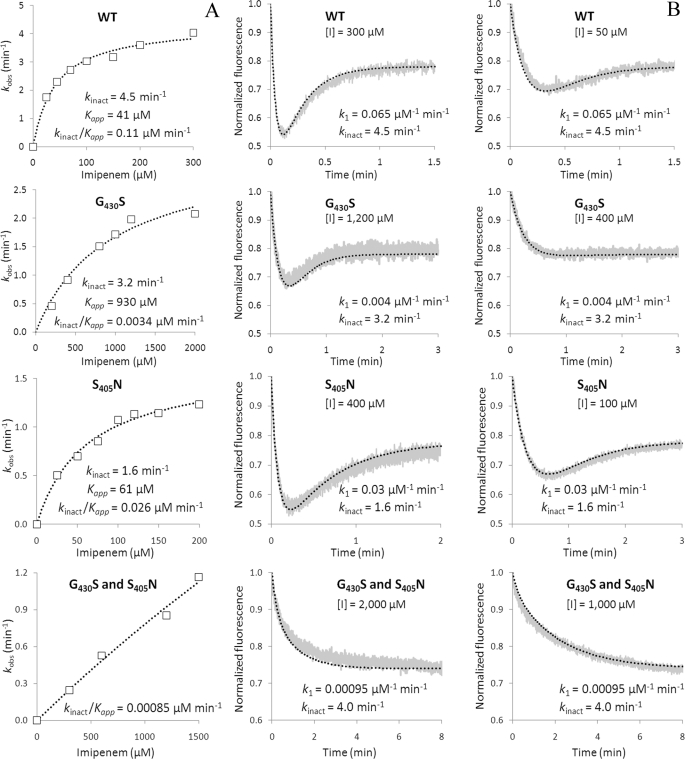
FIGURE 7.
Impact of amino acid substitutions S405N and G430S on the kinetics of Ldtfm inactivation by imipenem. A, determination of the catalytic constants kinact and Kapp by spectrophotometry. The values of kobs were plotted as a function of imipenem concentration [I], and regression analysis was performed using the equation, kobs = kinact[I]/Kapp + [I], in which kinact is the first-order constant for acylenzyme formation and Kapp is a constant. B, determination of the catalytic constants k1 by spectrofluorimetry. The charts present examples of kinetics at two different concentrations of imipenem. The gray and black curves correspond to normalized fluorescence for experimental data points and the simulations, respectively. The simulations were performed with the indicated values of k1 and kinact. A value of 0.1 min−1 was used for k−1 for all kinetics. WT, wild-type enzyme.