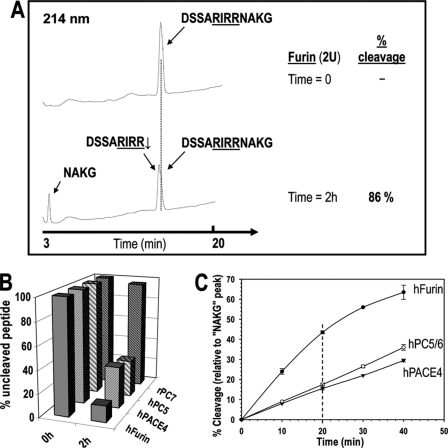
FIGURE 2.
In vitro, furin is better than PC5/6 or PACE4 at processing the 12-mer mouse BMP10 peptide at the predicted R311IRR314↓ cleavage site, whereas PC7 does not cleave this peptide. A, typical RP-HPLC profile for in vitro digestion of the 12-mer mouse BMP10 peptide with soluble furin. The synthetic peptide (200 μm) was incubated for 2 h in vitro with 2 units of purified soluble furin, PC5/6, PACE4, or PC7, as described under “Experimental Procedures.” The products were separated by RP-HPLC on a Varian C18 column (5 μm, 100 Å, 4.6 × 250 mm). The cleavage site RIRR↓ was confirmed by MS/MS. The % cleavage was calculated as the ratio of the normalized peak areas (peak area/number of peptide bonds) of C-terminal fragment NAKG and the intact 12-mer peptide (at time 0). B, summary of the in vitro 2-h digestions of the 12-mer mBMP10 peptide with furin, PC5/6, PACE4, and PC7, respectively, based on RP-HPLC analyses. The results represent an average of two independent experiments. C, time dependent in vitro cleavage of the 12-mer mBMP10 peptide. The synthetic peptide was incubated with purified PCs in vitro for variable amounts of time. For each time point, the incubation mixture was subjected to RP-HPLC separation and the % cleavage was calculated and plotted as a function of time. Averages of two independent experiments are presented. Based on the linear range of the respective rate profiles (e.g. % cleavage at 20 min), the 12-mer mBMP10 peptide is a ∼3-fold better substrate for furin than for PC5/6 or PACE4.