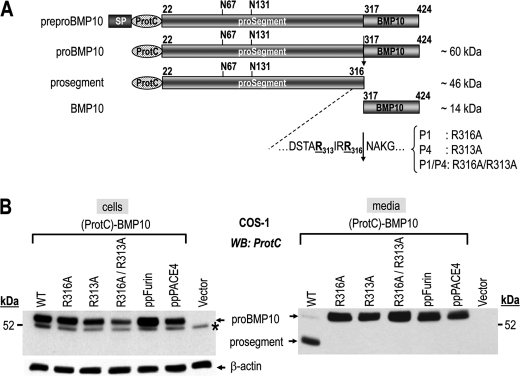
FIGURE 3.
Ex vivo validation of the R313IRR316↓ cleavage site by site-directed mutagenesis. A, schematic representation of the 424-aa human prepro-BMP10 and its derived forms, pro-BMP10, prosegment, and mature BMP10 (BMP10). Depicted are the signal peptide (SP), N-terminal ProtC tag, potential N-glycosylation sites (N67, N131), predicted PC-processing site (R313IRR316↓) and its mutants: P1 (R316A), P4 (R313A), and P1/P4 (R316A/R313A). B, cell lysates (left) and 20-h conditioned media (right) from COS-1 cells transiently expressing (ProtC)-BMP10 carrying either no mutation (WT; lane 1) or mutations R316A (lane 2), R313A (lane 3), and R316A/R313A (lane 4), or (ProtC)-BMP10 WT and either prepro-furin (ppFurin; lane 5) or prepro-PACE4 (ppPACE4; lane 6), or no protein (vector, lane 7) were analyzed by Western blotting using a rabbit ProtC-Ab. Processing of pro-BMP10 (pro) WT into its prosegment is detected only in the medium (right). The mutant forms of pro-BMP10 (R316A, R313A, and R316A/R313A) are no longer cleaved. In cell lysates only the uncleaved form (pro-BMP10) is detected (left). *, nonspecific band. The data are representative of at least two independent experiments. WB, Western blot.