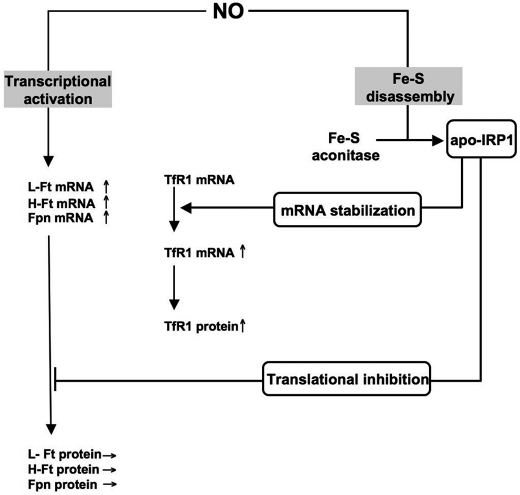
FIGURE 7.
Scheme of dual transcriptional and IRP1-dependent regulation of cellular iron metabolism in response to NO. In wild-type BMMs, NO is responsible for the transcriptional activation of the L-, H-Ft, and Fpn genes (left side). Nonetheless, up-regulation of the L-, H-Ft, and Fpn mRNAs does not finally lead to increased L, H-Ft, and Fpn protein levels. This is explained by the fact that NO mediates posttranslational modification of the aconitase/IRP1 system (right side). By rapidly converting the constitutively expressed cytosolic Fe-S aconitase into an iron regulatory factor (apo-IRP1), NO in fine inhibits translation of the L-, H-Ft, and Fpn mRNAs. This latter action of NO predominates on the former one because IRP1 Fe-S disassembly by NO is a fast process that does not require protein synthesis and that generates sufficient amount of apo-IRP1 to efficiently prevent translation of elevated levels of L-, H-Ft, and Fpn mRNAs. In an opposite way, TfR1 mRNA is stabilized by the NO dependent-IRP1 activation, leading to the increase in TfR1 protein levels.