FIGURE 4.
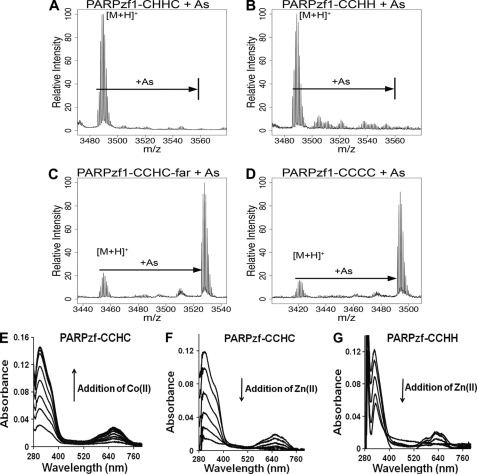
Arsenite binding selectivity for PARP-1-derived peptides. A–D, arsenite (As) binding to native and substituted PARPzf1 peptides was assessed by MALDI-TOF-MS analysis as described under “Experimental Procedures.” A, lack of arsenite binding to a PARPzf1-CHHC peptide mutant. B, lack of arsenite binding to a PARPzf1 peptide with a CCHH configuration that retains vicinal cysteine residues. C, arsenite binding to a PARPzf1-CCHC peptide with an insertion between the first two vicinal cysteine residues (CCHC-far) was detected at m/z = 3526. D, arsenite binding to a PARPzf1-CCCC peptide mutant was detected at m/z = 3491. E–G, cobalt spectrometry analysis of zinc binding to native and substituted PARPzf1 peptides. E, 100 μm native PARPzf1 peptide in 5 mm Tris (pH 7.4) was titrated with Co(II) over a range of 10–100 μm in increments of 10 μm. F, 100 μm native PARPzf1 peptide was saturated with 300 μm Co(II) and back-titrated with Zn(II) over a range of 10–50 μm in increments of 10 μm under argon. G, 100 μm mutant PARPzf1 peptide (CCHH) was saturated with 300 μm Co(II) and back-titrated with Zn(II) over a range of 10–40 μm in increments of 10 μm in each addition under argon. Similar results were obtained with each of the PARP-1 peptides shown in Fig. 3 (data not shown).