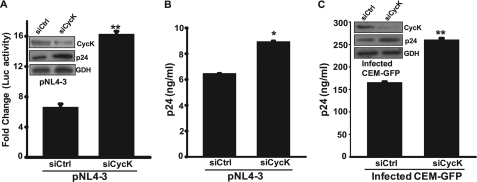
FIGURE 3.
Cyclin K silencing increases LTR-mediated gene expression and virus production in T-cells. A, inhibition of Cyclin K expression by siRNA leads to an increase in LTR-driven luciferase activity in Jurkat-1G5 cells. Jurkat-1G5 cells were transfected with 100 nm control (siCtrl) siRNA or CycK-specific siRNA (siCycK), and 24 h post-transfection, cells were transfected with pNL4-3 vector (1 μg). 48 h post-transfection, cells were harvested, and a luciferase assay was performed. The inset shows the efficiency of siRNA-mediated Cyclin K silencing by immunoblotting. B, inhibition of Cyclin K expression by siRNA leads to an increase in virus production from Jurkat-1G5 cells. The virus released in the culture supernatant of cells in the experiment described in A was estimated by a p24 gag antigen capture ELISA. C, Cyclin K silencing increases virus production in HIV-1NL4-3-infected CEM-GFP cells. CEM-GFP cells were first transfected with 200 nm control or CycK-specific siRNA. 24 h post-transfection, cells were infected with HIV-1NL4-3 at 0.5 multiplicity of infection, and on day 3, the culture supernatant was used for a p24 antigen capture ELISA. The inset shows the efficacy of siRNA-mediated silencing by immunoblotting. GDH, Glyceraldehyde-3-phosphate dehydrogenase loading control.