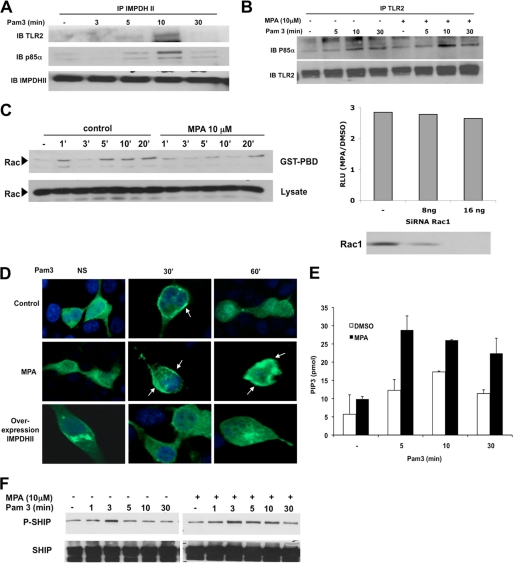
FIGURE 6.
IMPDHII is recruited to the TLR2-dependant activation cluster and negatively regulates PI3K activity. A, HEK293-T2 cells were stimulated with Pam3 (1 μg/ml). Cell lysates were immunoprecipitated (IP) with 3 μg of IMPDHII antibodies, and TLR2, the p85α subunit of PI3K, or immunoprecipitated IMPDHII was visualized by Western blot (IB). B, HEK293-T2 cells were incubated with MPA (10 μm) for 2 h and stimulated with Pam3 (1 μg/ml). Cell lysates were immunoprecipitated with 3 μg of anti-TLR2 monoclonal antibody. The P85α subunit of PI3K and immunoprecipitated TLR2 were revealed by Western blot. C, left panel, time-dependent activation of Rac was quantified by the presence of Rac-GTP detected by Rac PBD pulldown in HEK293-T2 treated with MPA or DMSO (control cells) and stimulated with Pam3. Right panel, HEK293-T2 cells were co-transfected with siRNA targeting Rac1 or non-interfering scramble siRNA and with 5× κB-luciferase promoter gene plasmid (40 ng/ml) and β-galactosidase expression vector (40 ng/ml). Cells were then incubated or not with MPA for 2 h and stimulated with Pam3 for 6 h; NF-κB-driven luciferase activity was measured and rationalized upon β-galactosidase expression. Results are expressed as arbitrary units corresponding to the MPA/DMSO ratio of NF-κB activation. D, HEK293-T2 cells were transfected with a fusion protein combining GFP and the PH domain of Akt. Transfected cells were either co-transfected with IMPDHII expression vector or incubated with MPA (10 μm). Cells were then stimulated with Pam3 (1 μg/ml) and fixed with paraformaldehyde. Cell nuclei were stained with DAPI. Recruitment of the construct to the membrane was observed by confocal microscopy for each condition. NS, nonstimulated. E, THP1 cells were incubated with MPA (10 μm) or DMSO for 2 h and then stimulated with Pam3 (1 μg/ml). PI3K was then immunoprecipitated with antibody against the p85α subunit and incubated with PI(4,5)P2. The reaction products were incubated with a PI(3,4,5)P3 detector protein and then added to a PI(3,4,5)P3-coated microplate for competitive binding. A peroxidase-linked secondary detection reagent and colorimetric detection buffer were used to detect PI(3,4,5)P3 detector protein binding to the plate. The amount of PI(3,4,5)P3 produced by PI3K activity was inversely proportional to the colorimetric signal. Data are the means ± S.D. representative of three independent experiments. F, THP1 cells were incubated with MPA (10 μm) and stimulated with Pam3 (1 μg/ml). Immunoblot was assessed on lysates to evaluate the phosphorylation of SHIP-1 on Tyr-1020, reflecting its activation state. Results are representative of two independent experiments.