FIGURE 7.
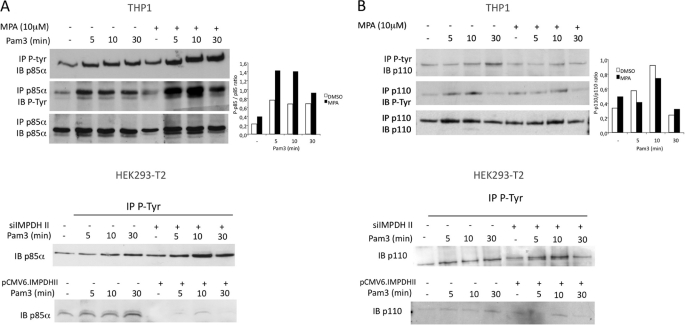
IMPDHII modulates tyrosine phosphorylation of p85α regulatory subunit of PI3K. A, upper panel, THP1 monocyte cells were incubated or not with MPA for 2 h and stimulated with Pam3. Lysates were immunoprecipitated (IP) with either 3 μg of anti-phosphotyrosine (P-Tyr, 4G10 clone) or anti-p85α antibodies, and precipitates were then immunoblotted with p85α or P-Tyr antibodies. Densitometry quantification of the phosphorylated form of p85α was performed using ImageJ software and normalized with the densities of precipitated p85α for each condition. Lower panel, HEK293-T2 cells were transfected either with siRNA targeting IMPDHII or with IMPDHII expression vector. Control conditions correspond to non-interfering scramble siRNAs and empty pCMV6 vector, respectively. Cells were then stimulated with Pam3, lysed, and immunoprecipitated with 3 μg of anti-phosphotyrosine antibodies (4G10 clone). The p85α subunit of PI3K was revealed by Western blot (IB). B, upper panel, THP1 lysates were immunoprecipitated with 3 μg of either P-tyrosine (4G10 clone) or p110 antibodies, and precipitates were then immunoblotted with anti-p110 or anti-4G10 antibodies. Densitometry quantification of the phosphorylated form of p110 was performed using ImageJ software and normalized with the densities of precipitated p110 for each stimulated condition. Lower panel, HEK293-T2 cells were transfected either with siRNA targeting IMPDHII or with IMPDHII expression vector. Control conditions correspond to non-interfering scramble siRNAs and empty pCMV6 vector, respectively. Cells were then stimulated with Pam3, lysed, and immunoprecipitated with 3 μg of anti-phosphotyrosine antibodies (4G10 clone). The p110 catalytic subunit of PI3K was revealed by Western blot. Results are representative of three independent experiments.