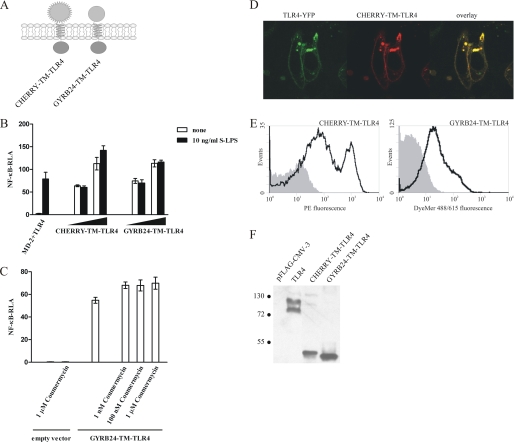
FIGURE 2.
Replacement of the TLR4 ectodomain by monomeric protein domains leads to the constitutive activation. A, schematic representation of the CHERRY-TM-TLR4 and GYRB24-TM-TLR4 chimeras. B, the CHERRY-TM-TLR4 and GYRB24-TM-TLR4 chimeric constructs constitutively activate target promoters. HEK293 cells were transiently transfected with reporter plasmids as well as increasing amounts (1 ng and 10 ng) of plasmid DNA encoding the CHERRY-TM-TLR4 or GYRB24-TM-TLR4 expression constructs or with MD-2- and TLR4-encoding plasmids (1 ng each). After 18 h of stimulation with LPS, luciferase activity (RLA) was measured in the cell lysates. C, addition of coumermycin A1 to GYRB24-TM-TLR4-expressing cells does not enhance cell activation. HEK293 cells were transiently transfected with reporter plasmids as well as with 5 ng of plasmid DNA encoding the GYRB24-TM-TLR4 construct or an empty vector. After 18 h of stimulation with different amounts of coumermycin A1, luciferase activity was measured in the cell lysates. D and E, the CHERRY-TM-TLR4 and GYRB24-TM-TLR4 chimeric constructs are expressed at the cell surface. D, HEK293 cells were transiently cotransfected with a plasmid encoding TLR4-YFP and a plasmid encoding the CHERRY-TM-TLR4 chimera (80 ng each). The expression profiles of both constructs was compared using a confocal microscope 36 h post-transfection. E, HEK293T cells were transiently transfected with 2 μg of CHERRY-TM-TLR4 or GYRB24-TM-TLR4 plasmid DNA. Cell surface expression levels of the constructs were determined by flow cytometric analysis 48 h after transfection. Histograms of mock-transfected cells (filled areas) or cells transfected with the appropriate DNA (solid lines) are shown. F, expression of chimeric constructs in HEK293T cells. HEK293T cells were transiently transfected with 2 μg of MD-2-TM-TLR4, MD-2-(4CYS)-TM-TLR4, CD14-TM-TLR4, CD14-(4CYS)-TM-TLR4, or TLR4 plasmid DNA. 48 h post-transfection, cell lysates were prepared, and 30 μg of total proteins were loaded onto an SDS-PAGE gel and blotted using anti-FLAG antibody. Molecular weight marker sizes are given in kDa.