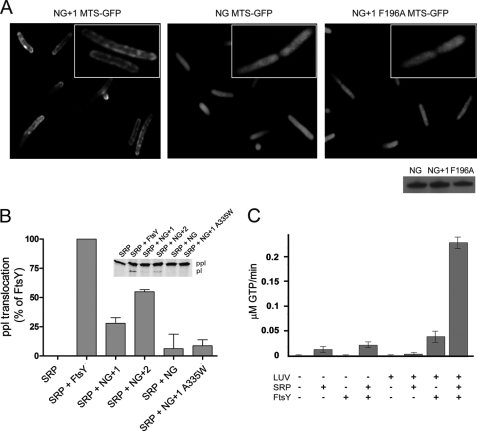
FIGURE 5.
MTS is functional in vivo and in vitro. A, fluorescence micrographs of the localization of the MTS of NG+1 (left), NG (middle), and NG+1 F196A (right) fused to the N terminus of GFP. The inset shows the magnification of a representative E. coli cell. The Western blot shows that all MTS-GFP fusions were expressed in E. coli at similar levels after 1 h of induction. B, ability of FtsY, NG+1, NG+2, and NG to support membrane translocation of the SRP model substrate ppl. The inset shows the SDS-PAGE used to separate ppl and prolactin (pl). The efficiency of ppl translocation was quantified and is given in percent relative to FtsY. The error bars represent the standard deviation between three independent measurements. An FtsY variant that contains a functional MTS but is defective in reciprocal GTPase activation in the context of SRP (NG+1 A335W variant) (26) was used as a control and shows a similar translocation defect as observed for NG. C, anionic phospholipids stimulate both the basal GTPase activity of FtsY and its complex assembly with SRP.