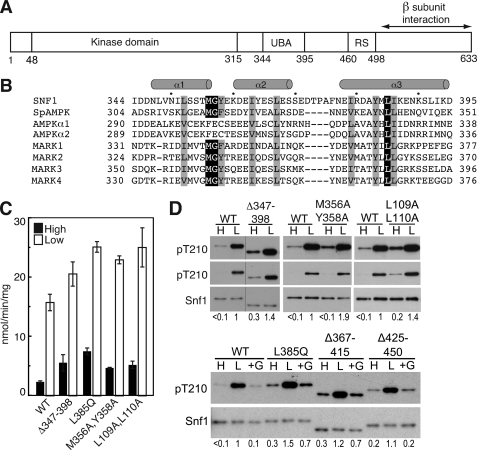
FIGURE 1.
Effects of the Snf1 UBA domain on Thr210 phosphorylation. A, schematic representation of Snf1 catalytic subunit. UBA, UBA domain. RS, regulatory sequence that interacts with Snf4 in the crystal structure (30). B, sequence alignment of UBA and UBA-like domains of the catalytic subunits of SNF1, S. pombe (Sp) AMPK, and human AMPKs and MARKs. Residues are indicated in single-letter code. Conserved hydrophobic residues are highlighted. Secondary structure designation is based on Chen et al. (39). C and D, wild-type (WT) and mutant Snf1 proteins were expressed in snf1Δ cells. Cells were collected after growth in 2% (high, H) glucose (filled bars) or after a shift to 0.05% (low, L) glucose for 10 min (open bars), as described under “Experimental Procedures.” In some cases, after incubation in 0.05% glucose, an aliquot of cells was incubated in SC plus 2% glucose (+G) for 10 min and collected. C, assays of SNF1 catalytic activity. D, immunoblot analysis to detect phosphorylated Thr210 (pT210) and Snf1 protein. Two exposures for detection of pT210 are shown in the upper panels. The bar indicates that lanes were from the same blot, but not adjacent. Immunoblot analysis was carried out on three independent transformants expressing each mutant protein; representative samples are shown. Values indicate the relative intensity of the bands corresponding to phosphorylated Snf1-Thr210 and total Snf1 protein, normalized to a value of 1 for WT in low glucose. For the upper panels, values were determined using the shorter exposures; for Snf1M356A,Y358A on high glucose, the longer exposure gave a value that was 4-fold greater than that for WT.