FIGURE 1.
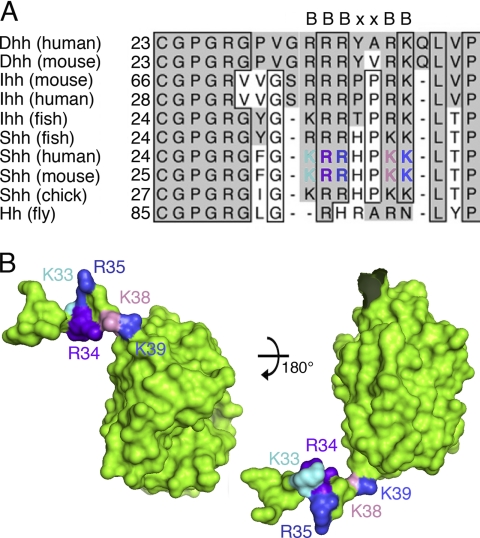
The heparin-binding Cardin-Weintraub sequence is highly conserved and located at the Shh N terminus. A, comparison of the amino acid sequences of mammalian N-terminal Hh peptides and Drosophila Hh reveals the presence of two highly conserved motifs: a block of residues required for N-terminal acylation (25), ranging from 25 to 30 (mouse Shh), and the highly conserved CW sequence (where B represents a basic residue) (17). Boxes represent absolutely conserved amino acid residues in at least eight gene products, gray columns indicate the presence of conserved amino acids. B, structure of the CW sequence as revealed by the modeled murine ShhN crystal structure (template: Protein Data Bank entry 3M1N). The position of CW sequences located within the N-terminal extended peptides is indicated. Note that residues Arg34 and Lys38 are located on one side of the N-terminal peptide, and residues Lys33, Arg35, and Lys39 are located on the opposite side. Color coding of basic CW residues is as in A.