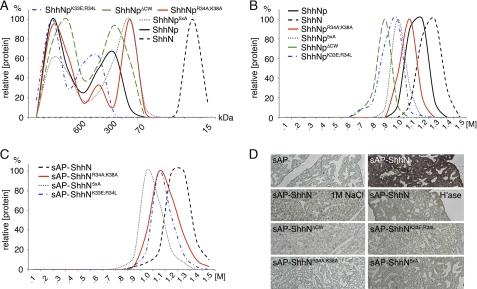
FIGURE 2.
Properties of CW mutant and wild-type Shh proteins. A, gel filtration analysis of supernatant derived from full-length ShhNp-expressing Bosc23 cells reveals the presence of “large” (>600 kDa) and “small” (70–600 kDa) multimers. In contrast, ShhN was exclusively monomeric. Site-directed mutagenesis of the Cardin-Weintraub sequence did not abolish ShhNp multimerization but affected the generation of large and small clusters to varying degrees. Representative results from one of three independently conducted experiments are shown. Molecular sizes of molecular weight standards are indicated. B, protein-heparin interactions of wild-type and CW mutant ShhNp as determined by heparin affinity chromatography. All multimeric CW mutant morphogens bind strongly to heparin. Recombinant, soluble ShhNp eluted at high salt concentrations (1–1.3 m NaCl), indicating strong ShhNp-heparin interactions. ShhN, however, showed even higher heparin affinity, eluting at 1.1–1.4 m NaCl. Reduced binding (>0.6 m NaCl) was observed for all mutant forms. C, heparin affinity chromatography of sAP-tagged ShhN monomers. Equal amounts of the wild-type and mutant morphogens were applied to the column, as assessed by sAP activity determined in the medium. Notably, all morphogens eluted at comparable salt concentrations from heparin. The heparin binding capacity was also comparable; integration of elution curves revealed 6.53, 5.12, 5.57, and 7.1 arbitrary units for sAP-ShhN, sAP-ShhNK33E/R34L, sAP-ShhNR34A/K38A, and sAP-ShhN5xA, respectively. D, ligand binding assay. Paraformaldehyde-fixed embryonic lung sections were treated with equal amounts of sAP alone, sAP-ShhN, and sAP-ShhN in the presence of 1 m NaCl, or tissues were pretreated with heparinase I–III to remove cell surface HS. Bound morphogens were detected via sAP activity upon incubation with NBT-BCIP. sAP-ShhN bound to HS in tissues, but CW mutants did not.