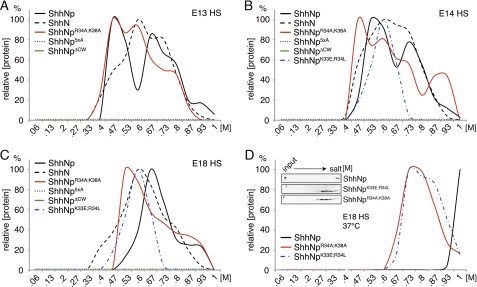
FIGURE 4.
CW mutant ShhNp multimers differ in their HS binding capacities and HS binding strength. Wild-type and mutant ShhNp were expressed in Bosc23 cells. The supernatant was filtered and applied to the same E13, E14, and E18 embryonic HS-coupled columns, and bound proteins were eluted with salt (0–1 m). ShhNp detected in the eluate was immunoblotted, and signals were quantified by ImageJ. The strongest signal was set to 100%. A, ShhNp and ShhNpR34A/K38A both bound E13 HS. In contrast, ShhNp5xA and ShhNpΔCW showed no detectable binding. ShhN served as a control. B, ShhNp5xA and ShhNpΔCW showed no detectable binding to E14 HS; however, ShhNpK33E/R34L and ShhNpR34A/K38A bound to E14 HS. ShhNp eluted at 0.63 and 0.73 m salt, and ShhNpR34A/K38A showed elution peaks around 0.47, 0.6, 0.73, and 0.93 m NaCl. In contrast, ShhNpK33E/R34L displayed a single elution peak at 0.6 m NaCl. C, ShhNp binding to E18 HS differed from both previous profiles. The relative amount of strong binding sites was increased over weak binding sites, and a fraction of the wild-type multimer, like the monomeric form, eluted at 0.93 m NaCl. ShhNp5xA and ShhNpΔCW elution was not detected, and most ShhNpR34A/K38A bound only weakly to HS (0.53 m NaCl). ShhNpK33E/R34L again displayed only one (low affinity) peak eluting at 0.6 m NaCl. D, at physiological temperature, ShhNp showed increased HS interaction (eluting at 0.8–1 m NaCl), indicating cooperative and temperature-dependent binding to E18 HS. Notably, both ShhNpK33E/R34L and ShhNpR34A/K38A also eluted at higher salt concentrations. Inset, immunoblotted input and eluates, demonstrating high capacity ShhNp binding to E18 HS. In contrast, ShhNp5xA and ShhNpΔCW binding to E18 HS were not observed.