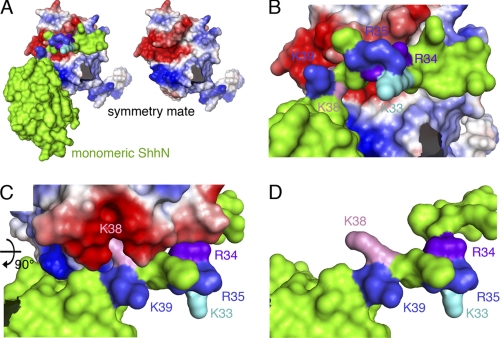
FIGURE 6.
Intermolecular interactions observed in the human ShhN crystal structure (Protein Data Bank entry 3M1N) suggest CW interaction with adjacent molecules in the secreted morphogen cluster. Analysis of intermolecular electrostatic interactions as revealed by the human ShhN crystal structure suggests protein-protein contacts involving extended N-terminal peptides of symmetry-related molecules in the crystal. One subunit is colored green, the interacting molecule is colored according to electrostatic potential (red, negatively charged; blue, positively charged). A, the N-terminal CW motif interacts with a stretch of negatively charged residues present on the adjacent morphogen in the cluster (right). B and C, detailed view of the interaction site. CW residues Lys33, Arg35, and Lys39 (mouse nomenclature) are surface exposed, whereas residues Arg34 and Lys38 make contact with the negatively charged patch on the adjacent molecule. D, same orientation as C, with the interacting molecule removed for better visualization of residues Arg34 and Lys38.