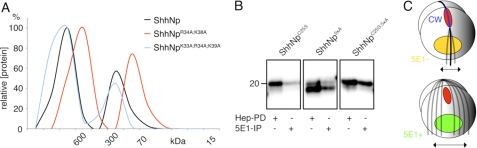
FIGURE 9.
CW residues 34 and 38 interact with adjacent molecules in the secreted morphogen cluster. A, gel filtration analysis of ShhNp, ShhNpR34A/K38A, and ShhNpK33A/R35A/K39A. ShhNpR34A/K38A multimer sizes were reduced, and the relative amounts of smaller clusters were increased, consistent with the suggested contribution of the two residues to protein-protein interactions. B, mutant and wild-type morphogens were subjected to immunoprecipitation using 5E1 coupled to protein A-Sepharose, and the immune precipitates were subjected to SDS-PAGE and immunoblotting. For normalization of different expression levels, heparin-Sepharose pull-downs of the same expressions served as controls. Soluble ShhNp5xA represents an N-terminally truncated morphogen (29), thereby exposing an epitope recognized by the 5E1 antibody (positive control). In contrast, ShhNpC25S is always released in non-truncated form, preventing binding of the 5E1 antibody to its (blocked) epitope (negative control). 5E1 binding is restored in a double mutant, untruncated form, suggesting increased spatial flexibility of the N-terminal peptide in the CW mutant morphogen. Note that ShhNpC25S shows a size comparable with that of ShhNpC25S/5xA. This indicates that both morphogens were released in N-terminally unprocessed form. C, model of CW-dependent spatial fixation of N-terminal peptides in the cluster. Functional inactivation of the CW motif (bottom) increases the spatial degree of freedom of the N-terminal peptide, resulting in increased accessibility of the 5E1 epitope.