Abstract
Puillandre, N. et al. (2010) Genetic divergence and geographic variation in a deep-water cone lineage: molecular and morphological analyses of the Conus orbignyi complex (Mollusca: Conoidea).
The cone snails (family Conidae) are a hyperdiverse lineage of venomous gastropods. Two standard markers, COI and ITS2, were used to define six genetically-divergent groups within a subclade of Conidae that includes Conus orbignyi; each of these was then evaluated based on their shell morphology. We conclude that three forms, previously regarded as subspecies of Conus orbignyi are distinct species, now recognized as Conus orbignyi, Conus elokismenos and Conus coriolisi. In addition, three additional species (Conus pseudorbignyi, Conus joliveti and Conus comatosa) belong to this clade. Some of the proposed species (e.g., Conus elokismenos) are possibly in turn complexes comprising multiple species. Groups such as Conidae illustrate the challenges generally faced in species delimitation in biodiverse lineages. In the case of the Conus orbignyi complex, not only are there definable, genetically divergent lineages, but also considerable geographic variation within each group. Our study suggests that an intensive analysis of multiple specimens within a single locality helps to minimize the confounding effects of geographic variation and can be a useful starting point for circumscribing different species within such a confusing complex.
Keywords: COI gene, Conidae, ITS2 gene, Phylogeny, Species delimitation
Introduction
The cone snails (family Conidae) comprise a hyper-diverse group of venomous gastropods. Because the peptides produced by their venom gland have medical potential (Terlau & Olivera, 2004; Olivera, 2006), there has been intense recent interest in this biodiverse lineage. Conus orbignyi Audouin, 1831 (Bathyconus orbignyi in the Tucker & Tenorio, 2009 classification) is one of the problematic species complexes in the Conidae; traditionally Röckel et al., 1995, it is regarded as a widely-distributed species ranging from the Southwest Pacific, to Southwest Africa and north to Honshu, Japan where it is one of the few species in Conidae that thrives at such northern latitudes. In the 19th and first half of the 20th Century, most specimens were collected by Japanese or Chinese fishing boats. The holotype from China is an example of the Northern Pacific form of Conus orbignyi.
Specimens assigned to Conus orbignyi from South Africa, Mozambique and Madagascar are generally regarded as a geographic subspecies, Conus orbignyi elokismenos Kilburn, 1975. In the Coral Sea - New Caledonia area, a form known as Conus orbignyi coriolisi Moolenbeek & Richard, 1995 has been described. There are two species that also are somewhat nodulose that appear to be closely related to Conus orbignyi: one is Conus pseudorbignyi Röckel & Lan, 1981, described from Taiwan, and the other is Conus joliveti Moolenbeek, Röckel & Bouchet, 2008, from Fiji. Because cones are a collectable group of seashells, many of the recently named species, especially those based on a small number of specimens, are sometimes viewed with suspicion by other biologists, and admittedly the taxonomy of C. orbignyi, as well as its affinities to morphologically-similar species has not been thoroughly assessed by non-typological approaches.
The variability of mollusc shells, and in particular Conoidea shells, is known to be difficult to interpret (e.g. Duda et al., 2008, Puillandre et al., 2010), due to extended polychromatism and rampant homoplasy. The variability of molluscan shells, especially in the case of small samples, is difficult to attribute a priori to individual variability, within species geographic variation or differences between species (see e.g. Meyer et al., 2005; Appleton & Palmer, 1988). Molecularly well-defined species may have very subtle conchological differences, but in the meantime morphologically distinct clusters of specimens could correspond merely to geographical variants, with different environmental conditions acting differentially on the shape and ornamentation of the shell. Consequently, investigating whether these forms correspond to different species or not requires analysis of characters not determined by the environment. DNA sequences meet this requirement, and are now commonly used to delimit species (e.g. Wiens, 2007; Wheeler, 2009). In the genus Conus, Duda et al. (2008) recently published a DNA-based analysis of the species diversity in the C. sponsalis complex, another complex in the Conidae where morphological characters alone were unable to accurately define species limits. Several other studies have also been carried out on Conus-related groups belonging to the superfamily Conoidea (e.g. Puillandre et al., 2009, 2010).
We present a similar approach to the orbignyi complex, analyzing first DNA variation to delimit putative species, then morphological variation to link these putative species to the different forms described in literature. Although our analysis includes specimens collected in other geographical regions, we focus on forms related to C. orbignyi from the Philippines, as the starting point of this research was an expedition carried out at Aurora, Luzon Island in 2007 (“Aurora 2007”) that recovered over 100 specimens that could be assigned to the Conus orbignyi complex.
We applied several species delimitation criteria in the analysis of our C. orbignyi dataset (see e.g. De Queiroz, 1998, 2007; Samadi & Barberousse, 2006). First, specimens that belong to the same species are supposed to be more similar to each other than to any other species; we used the COI gene to delimit groups of specimens. Then, phylogenetic analyses were carried out not only using the COI gene, but also the 12S and 16S genes to establish the monophyly of each group. However, discrepancies between gene tree and species tree can occur, due, for example, to mtDNA introgression or incomplete lineage sorting (Funk & Omland, 2003; Maddison & Knowles, 2006; Linnen & Farrell, 2008; Petit & Excoffier, 2009). To overcome this difficulty, an independent nuclear marker ITS2 was assessed: if the phylogenetic relationships inferred with each gene are congruent at the interspecific level (i.e. between putative species), then interspecific gene trees can be equated to the species tree (Gaines et al., 2005; Knowles & Carstens, 2007; Edwards, 2009).
Finally, an attempt was made to define morphological differences between the genetic groups, taking into account geographical variation.
Material and Methods
Alcohol preserved specimens
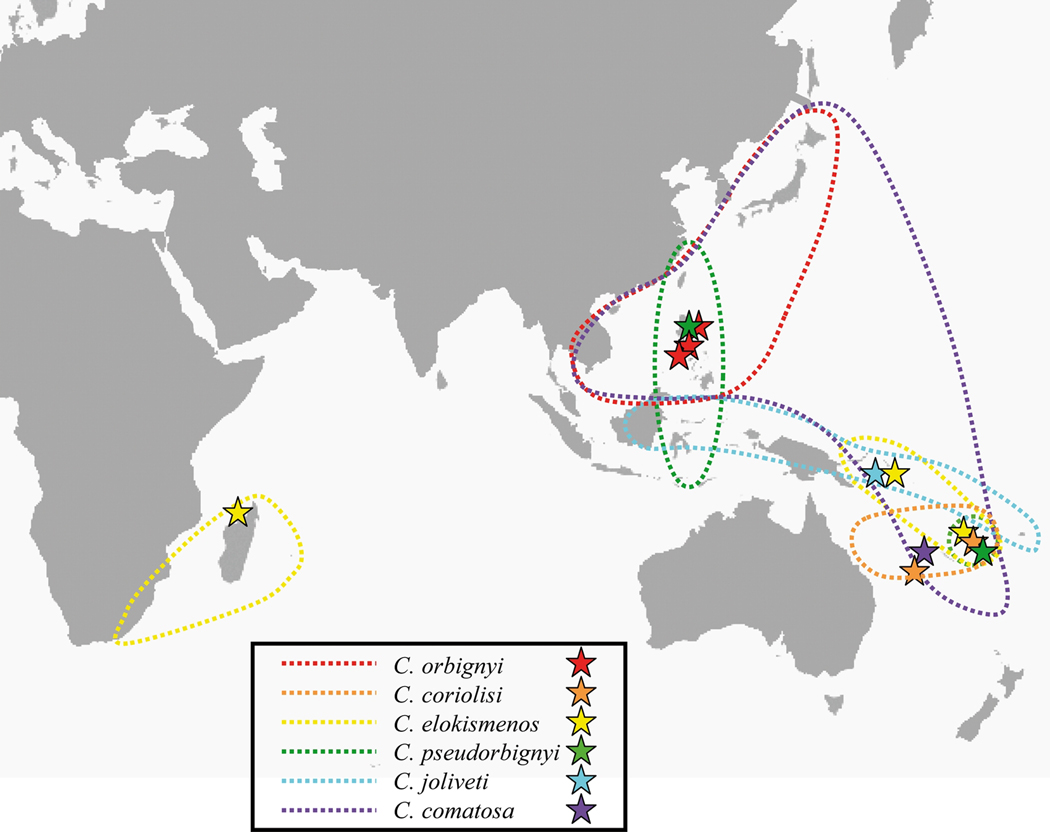
Specimens morphologically identified as belonging or closely related to the C. orbignyi complex were collected in Taiwan, Philippines, Solomon and Chesterfield Islands, Vanuatu and Madagascar during several expeditions conducted between 2003 and 2009 (Table 1). The known geographic distribution of the different species, together with the sampling sites, is shown in the Fig. 1. A piece of foot was cut from the living animal and preserved in alcohol 95% for molecular analyses, and the corresponding shell was kept for further reference. For the phylogenetic analyses, three species were used as outgroups: C. mahogani (Ximenoconus mahogani in the Tucker & Tenorio, 2009 classification), C. vimineus (Viminiconus vimineus in the Tucker & Tenorio, 2009 classification) and C. ichinoseanus (Yeddoconus ichinoseanus in the Tucker & Tenorio, 2009 classification). Within Conus, all are included in the small major clade (sensu Duda & Kohn 2005). C. vimineus and C. ichinoseanus are placed together with the C. orbignyi complex in Conolithinae by Tucker & Tenorio (2009). With the exception of a Taiwan specimen housed at FLMNH, all specimens are vouchered in MNHN and registered in the Barcode Of Life Database (Table 1).
Table 1.
List of specimens analysed molecularly, with collection data, species identification and GenBank and BOLD (Barcode Of Life Database) numbers.
| ID | Geographic region | Expedition | Coordinates and depth (m) | Group | Species | GenBank numbers | BOLD ID | |||
|---|---|---|---|---|---|---|---|---|---|---|
| COI | ITS2 | 16S | 12S | |||||||
| MNHN IM200730681 | Chesterfield | EBISCO | 21°05'S, 160°45'E - 297–378 | 1 | comatosa | GU131299 | GU131322 | GU131286 | GU131274 | FRANZ906-09 |
| MNHN IM200730660 | Vanuatu | Santo 2006 | 15°39'S, 167°01'E - 266–281 | 2 | coriolisi | GU131297 | GU131320 | GU131285 | GU131273 | FRANZ907-09 |
| MNHN IM200730666 | Vanuatu | Santo 2006 | 15°39'S, 167°01'E - 266–281 | 2 | coriolisi | GU131298 | GU131321 | FRANZ908-09 | ||
| MNHN IM200730823 | Vanuatu | Santo 2006 | 15°40'S, 167°02'E - 272–286 | 2 | coriolisi | GU131306 | GU131328 | FRANZ909-09 | ||
| MNHN IM200730836 | Chesterfield | EBISCO | 21°06'S, 158°36'E - 356–438 | 2 | coriolisi | GU131307 | GU131329 | FRANZ910-09 | ||
| MNHN IM200730842 | Chesterfield | EBISCO | 21°06'S, 158°36'E - 356–438 | 2 | coriolisi | GU131308 | GU131330 | FRANZ911-09 | ||
| MNHN IM200730899 | Philippines | Aurora 2007 | 15°54'N, 121°42'E - 189–189 | 3 | pseudorbignyi | GU131312 | GU131333 | GU131289 | GU131277 | FRANZ912-09 |
| MNHN IM200730942 | Vanuatu | BOA 1 | 15°07’S, 166°54’E - 131–308 | 3 | pseudorbignyi | GU131316 | GU131337 | GU131292 | GU131280 | FRANZ913-09 |
| MNHN IM200730925 | Solomon Is. | Salomon 2 | 8°38’S, 157°22’E - 195–197 | 4 | joliveti | GU131313 | GU131334 | GU131290 | GU131278 | FRANZ914-09 |
| UF 327736 | Taiwan | 5 | orbignyi | GU131317 | GU131293 | GU131281 | ||||
| MNHN IM200717921 | Philippines | Panglao 2005 | 9°29'N, 123°44'E - 271–318 | 5 | orbignyi | EU015721 | CONO296-08 | |||
| MNHN IM200730729 | Philippines | Panglao 2005 | 9°39’N, 123°48’E - 255–268 | 5 | orbignyi | GU131301 | GU131324 | GU131288 | GU131276 | FRANZ915-09 |
| MNHN IM200730773 | Philippines | Panglao 2005 | 8°43’N, 123°19’E - 259–280 | 5 | orbignyi | GU131302 | GU131325 | FRANZ916-09 | ||
| MNHN IM200730785 | Philippines | Aurora 2007 | 16°03’N, 121°53E - 189-189 | 5 | orbignyi | GU131303 | GU131326 | FRANZ917-09 | ||
| MNHN IM200730813 | Philippines | Aurora 2007 | 16°03’N, 121°53E - 189-189 | 5 | orbignyi | GU131304 | GU131327 | FRANZ918-09 | ||
| MNHN IM200730815 | Philippines | Aurora 2007 | 16°03’N, 121°53E - 189-189 | 5 | orbignyi | GU131305 | FRANZ919-09 | |||
| MNHN IM200730714 | Vanuatu | BOA 1 | 15°05’S, 166°54’E - 400-350 | 6 | elokismenos | GU131300 | GU131323 | GU131287 | GU131275 | FRANZ920-09 |
| MNHN IM200730846 | Vanuatu | Santo 2006 | 15°07'S, 166°53'E - 328–354 | 6 | elokismenos | GU131309 | GU131331 | FRANZ921-09 | ||
| MNHN IM200730848 | Vanuatu | Santo 2006 | 15°07'S, 166°53'E - 328–354 | 6 | elokismenos | GU131310 | GU131332 | FRANZ922-09 | ||
| MNHN IM200730893 | Vanuatu | BOA 1 | 15°05’S, 166°54’E - 400-350 | 6 | elokismenos | GU131311 | FRANZ923-09 | |||
| MNHN IM200730938 | Vanuatu | BOA 1 | 15°05’S, 166°54’E - 400-350 | 6 | elokismenos | GU131314 | GU131335 | FRANZ924-09 | ||
| MNHN IM200730939 | Solomon Is. | Salomon 2 | 8°41’S, 157°24’E - 248–253 | 6 | elokismenos | GU131315 | GU131336 | GU131291 | GU131279 | FRANZ925-09 |
| MNHN IM20097513 | Madagascar | Miriki 2009 | 12°41'S, 48°17'E - 231–237 | 6 | elokismenos | GU131318 | GU131338 | GU131294 | GU131282 | FRANZ926-09 |
| OUTGROUPS | ichinoseanus | GU131319 | GU131296 | GU131284 | ||||||
| mahogani | FJ868119 | FJ868057 | FJ868049 | |||||||
| vimineus | GU134378 | EU682306 | EU682297 | |||||||
Fig. 1.
Known geographic distribution and sampling sites of the six delimited species.
DNA extraction and sequencing
Genomic DNA was extracted from a piece of foot or from hepatopancreas tissue, using the 6100 Nucleic Acid Prepstation system (Applied Biosystem), or the Gentra PUREGENE DNA Isolation Kit (Gentra Systems, Minneapolis, MN). Four genes were amplified: the “barcoding” fragment of the Cytochrome Oxidase I (COI) mitochondrial gene, using universal primers LCO1490 and HCO2198 (Folmer et al., 1994), the ITS2 gene, using primers mITS-3D and mITS-4R (Nam et al., 2009), a fragment of the 12S gene, using the primers 12S1 and 12S3 (Simon et al., 1991) and a fragment of the 16S gene, using the primers 16Sar and 16Sbr (Palumbi, 1996). All PCR reactions were performed in 25 µl, containing 3 ng of DNA, 10X reaction buffer, 2.5 mM MgCl2, 0.26 mM dNTP, 0.3 µM of each primer, 5% DMSO and 1 unit of Advantage 2 Polymerase Mix (Clontech Laboratories). Amplification consisted of an initial denaturation step at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 1min, annealing at 50°C for the COI gene, 61°C for the ITS2 gene, 54°C for 12S gene and 52°C for 16S for 30s, followed by extension at 68°C for 30sec. The final extension was at 60°C for 5min. PCR products were purified using the Qiagen gel extraction kit (Qiagen, CA, USA) or the High Pure PCR Product Purification Kit and sequenced by the Health Sciences Center Core Sequencing Facility, University of Utah. In most cases, both directions were sequenced to confirm accuracy of each haplotype sequence. All sequences were submitted to GenBank (Table 1).
Species delimitation using the COI gene
Specimens belonging to the same species are supposed to be phenetically similar. We used this property to delimit putative species within the orbignyi complex by sequencing a standard portion of the COI gene (the fragment used for most animals in DNA barcoding projects) for all alcohol-preserved specimens. Sequences were aligned manually, and the genetic p-distances were calculated between all sequences (excluding outgroups), using MEGA 3.1 (Kumar et al., 2004). Their distribution was visualized on a pairwise distances histogram, and groups of genetically similar specimens were proposed. A phylogenetic tree was then reconstructed using the COI gene dataset. The best model of evolution identified using the HLRT test implemented in Modelgenerator (Keane et al., 2006) is the TVM+I+G (with I = 0.37 and α = 0.23). Maximum likelihood analyses were performed using RaxML 7.0.4 (Stamatakis, 2006), with 20 independent runs, and the GTRGAMMAI model. Robustness of the nodes was assessed with 200 bootstrap replicates (with five searches for each of them). Bayesian analyses were performed using MrBayes (Huelsenbeck et al., 2001) and consisted of two independent analyses (eight Markov chains and five swaps at each sampling). A six substitution categories model associated to a gamma-distributed rate variation across sites and a proportion of invariable sites was used. Convergence of each analysis was evaluated using Tracer 1.4.1 (Rambaut & Drummond, 2007), and analyses were terminated when ESS values were all superior to 200. A consensus tree was then calculated after omitting the first 25% of the trees as burn-in.
ITS2 gene
To avoid problems linked to the use of a single gene in species delimitation, the ITS2 gene of several specimens for each putative species delimited with the COI gene was also sequenced. When specimens from different geographic region where included in a single putative species, the ITS2 gene of at least one specimen from each region was sequenced. Sequences were automatically aligned using BioEdit 7.0.5.3 (Hall, 1999). The best model of evolution for the ITS2 gene is TrN + G (α = 0.23). Phylogenetic relationships using the ITS2 gene were inferred following the methodology described for the COI gene.
Phylogenetic relationships between species
To infer the relationships between species, we also sequenced for several specimens representative of the COI variability of each putative species, two fragments of mitochondrial genes: the 12S and 16S. The two genes are less variable than the COI gene, and generally more informative to resolve deeper relationships. As for the ITS2 gene, sequences were aligned using Bioedit. The best models of evolution for the 12S and 16S genes are respectively HKY+I+G (with I = 0.47 and α = 0.21) and HKY+I+G (with I = 0.59 and α = 0.20). As the independent trees were congruent, we concatenated these two genes plus the COI gene in a single dataset. Five partitions were defined: three for each position of the COI gene, one for the 12S gene and one for the 16S gene. All partitions were unlinked, each following the GTRGAMMAI model (RAxML) or a model with six categories of substitution (MrBayes). Once again, phylogenetic relationships using these genes were then inferred following the methodology described for the COI gene.
Morphological analysis of living and dead specimens
Shells were a posteriori examined to identify differences between the different genetic groups. In addition to the living specimens analysed both molecularly and morphologically, several dead shells collected in the Philippines (and in particular during the Aurora 2007 expedition – Table 2) were integrated in the analysis. As all species and subspecies descriptions are based on shell characters, linking genetic groups to discrete morphological entities was the only way to attribute available taxon names to the different putative species of the orbignyi complex. The major character states used for differentiation were the ground color of the body whorl (white or brown), the banding pattern on the body whorl (number of darker brown bands, how continuous the bands are), how nodulose the spire sutural ridges are (very nodulose or almost obsolete) and the presence or absence of dark brown blotches between the spire nodules.
Table 2.
List of the Aurora 2007 expedition stations and number of dead shells for each Philippines species. (CP, beam trawl; DW, dredge)
| Station | Coordinates | Depth (m) | C. o. orbignyi | C. o. coriolisi | C. pseudorbignyi |
|---|---|---|---|---|---|
| CP2655 | 16°03N 121°53E | 189-189 | 6 | 3 | |
| CP2662 | 15°47N 121°44E | 253-253 | 1 | ||
| CP2666 | 15°57N 121°45E | 198-199 | 14 | 7 | |
| CP2672 | 14°57N 121°44E | 346-276 | 2 | ||
| CP2709 | 15°11N 121°35E | 296-244 | 6 | ||
| CP2710 | 15°15N 121°33E | 207-216 | 1 | ||
| CP2711 | 15°20N 121°32E | 200-184 | 3 | ||
| CP2712 | 15°21N 121°30E | 140-139 | 1 | ||
| CP2715 | 14°32N 121°42E | 233-249 | 10 | ||
| CP2716 | 14°31N 121°61E | 335-356 | 1 | ||
| CP2717 | 14°29N 121°42E | 361-311 | 17 | ||
| CP2719 | 14°27N 121°48E | 160-155 | 6 | ||
| CP2721 | 14°24N 121°47E | 367-360 | 4 | ||
| CP2723 | 14°25N 121°49E | 156-147 | 1 | ||
| CP2724 | 15°12N 121°35E | 280-229 | 2 | ||
| CP2741 | 16°03N 121°55E | 194-203 | 3 | ||
| CP2742 | 16°03N 121°53E | 182-205 | 3 | 2 | 1 |
| CP2748 | 15°56N 121°45E | 249-247 | 5 | ||
| CP2760 | 15°55N 121°41E | 100-100 | 1 | ||
| CP2762 | 15°52N 121°37E | 66-66 | 1 | ||
| DW2670 | 14°52N 121°49E | 180-187 | 3 | ||
| DW2726 | 15°04N 121°41E | 323-313 | 5 | ||
| DW2758 | 15°55N 121°50E | 173-151 | 1 | ||
| TOTAL | 93 | 12 | 5 |
Results
Molecular analyses
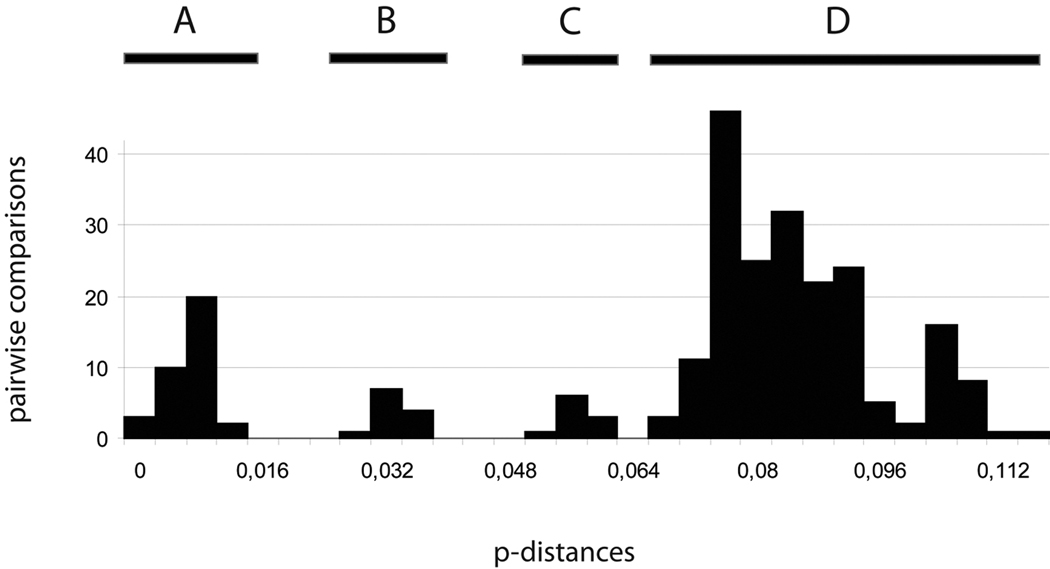
The 26 COI sequences alignment (23 ingroups and three outgroups) is 658 bp long. The pairwise p-distances range from 0 to 11.5% (excluding outgroups), and four modes can be recognized in the pairwise distribution (Fig. 2). This pattern is somewhat different from the classic bimodal distribution of pairwise genetic distance, with low distances corresponding to intraspecific distances and high genetic distances to interspecific distances (e.g. Hebert et al., 2004; Ward et al., 2005; Puillandre et al., 2010). The different thresholds between the four modes could be used to delimit groups of specimens in the COI tree (Fig. 3), but identifying which threshold between both corresponds to the species level is not straightforward.
Fig. 2.
Pairwise COI genetic p-distances between all the specimens. Four modes are indicated. A: Distances between specimens within group 2, 5 (Philippines specimens only) and 6 (Vanuatu specimens only); B. Distances within group 3, 5 (Taiwan vs Philippines) and 6 (Solomon vs Vanuatu); C. Distances within group 6 (Madagascar vs others – see text for discussion); D. Distances between groups.
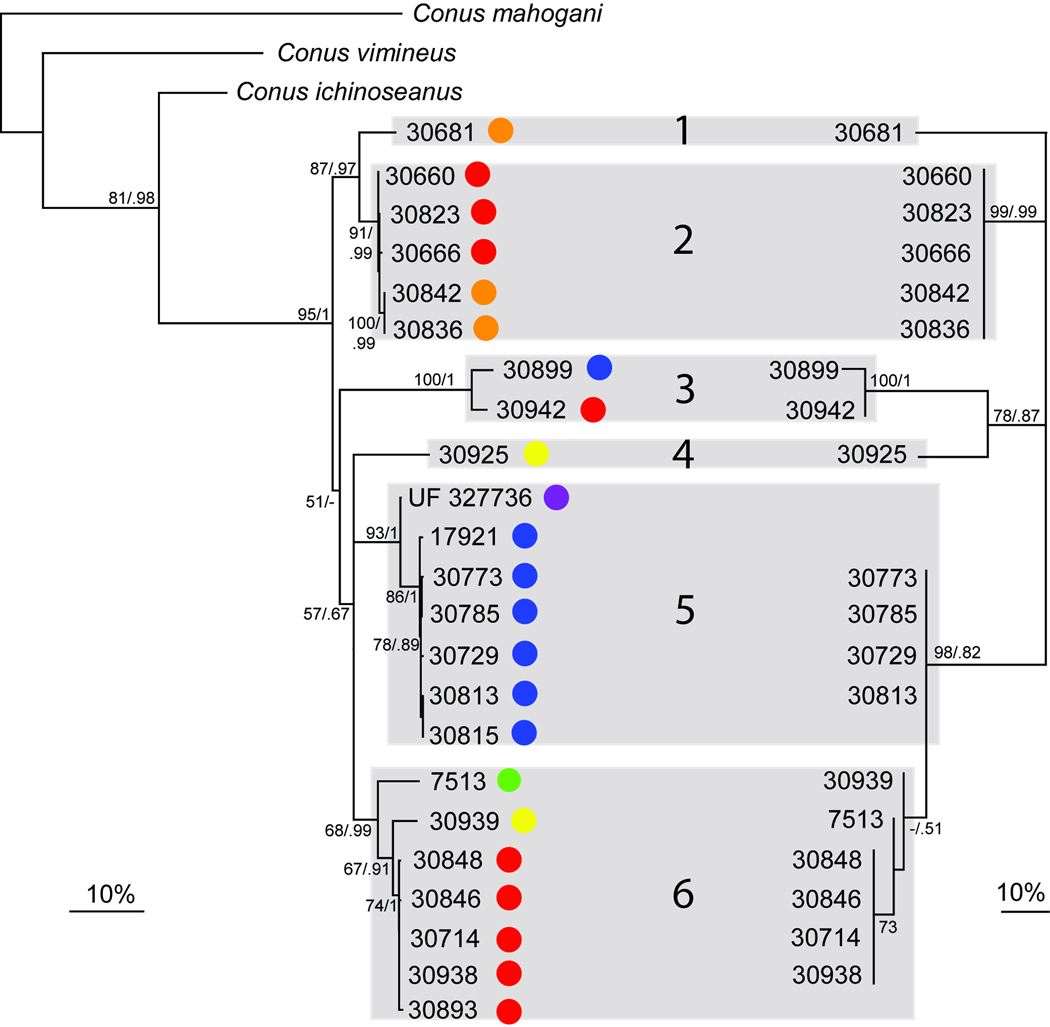
Fig. 3.
Left: COI phylogenetic tree (RAxML, best tree over 20 replicates), with bootstraps values (200 replicates) and posterior probabilities indicated for each node (when superior to 50 and 0.5 respectively). At the tip of each branch the geographic location is indicated (colors are used as in the Figure 1). Right: ITS2 phylogenetic tree (RAxML, best tree over 20 replicates), with bootstrap values (200 replicates) and posterior probabilities indicated for each node (when superior to 50 and 0.5 respectively). The congruency between both trees is highlighted with grey boxes, each of them representing a putative different species (groups 1 to 6).
The ITS2 gene was sequenced for 19 specimens, resulting in a 574 bp fragment after alignment. The ITS2 phylogenetic tree presented in Fig. 3 is highly congruent with the COI tree. All specimens placed in a single cluster using the third threshold defined by the four modes of the pairwise COI genetic distances distribution (< 6.4%), are characterized by highly similar ITS2 haplotypes (< 1% genetic divergence) and, conversely, specimens separated by more than 6.4% of divergence with the COI gene have different ITS2 haplotypes. Each of these groups (numbered from 1 to 6) correspond to highly supported monophyletic groups in both COI and ITS2 gene trees, except the distinction between Groups 5 and 6 in the ITS2 marker. Based on ITS2, Group 6 is a nested monophyletic clade within an undifferentiated Group 5.
Moreover, the identification of the threshold between intra and interspecific COI distances in comparison with the ITS2 gene tree allows an interpretation of the different modes found in the pairwise distribution shown in Fig. 2. The first (A) would correspond to intraspecific distances, mostly found between specimens collected in the same geographic region. The second mode (B) corresponds to distances between specimens collected in different geographic regions, but included in the same Group (3, 5, 6). The third mode (C) corresponds to distances between the specimen from Madagascar and the other specimens of Group 6 and the last mode (D) to distances between different groups.
Specimen MNHN IM20097513 from Madagascar is separated from the other specimens of Group 6 by 5 to 6% divergence in the COI gene.
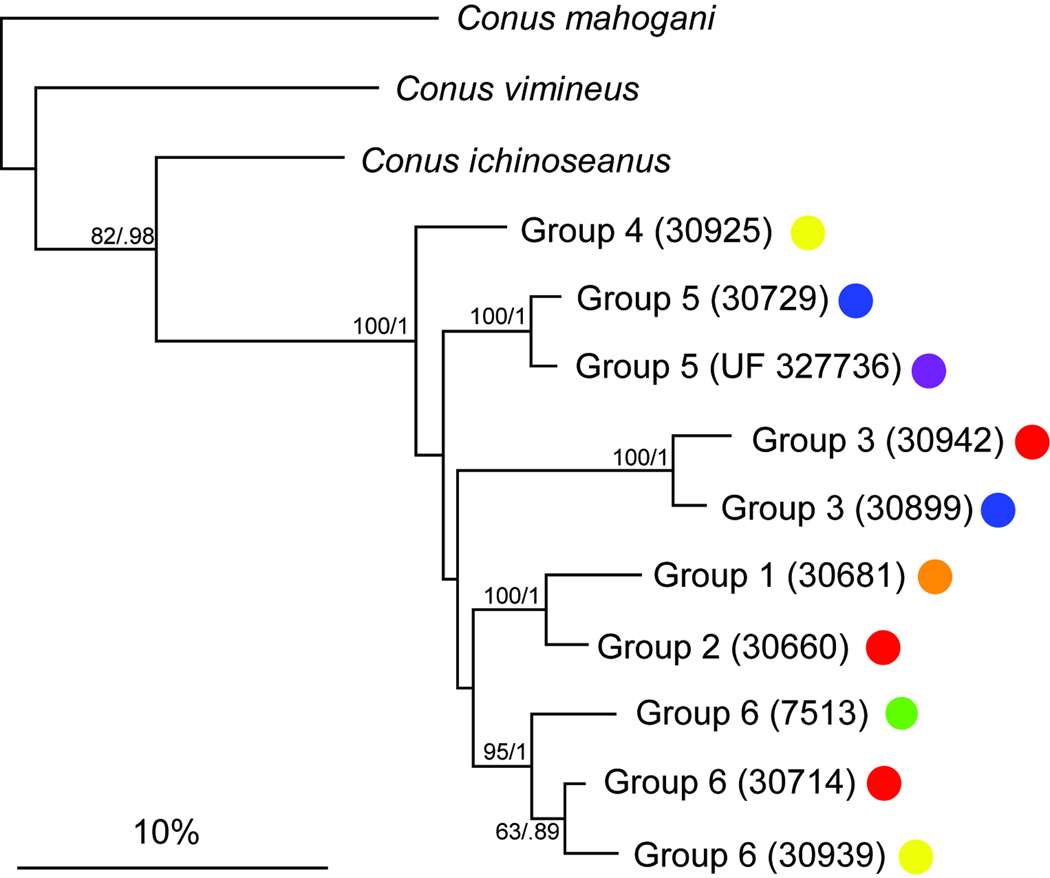
The 12S and 16S genes were used to infer the relationships between all the defined groups. The resulting fragments are 546 and 484 bp long respectively after alignment. In the resulting tree of the concatenated dataset (Fig. 4), the inclusion of one specimen from each geographic location within the Groups 3, 5 and 6 confirms their respective monophyly. As found with the COI and ITS2 genes, the C. orbignyi complex corresponds to a highly supported monophyletic group. However, most of the relationships between each group within this complex are not well supported.
Fig. 4.
Phylogenetic tree obtained with a concatenation of three genes (COI, 12S, 16S) with bootstrap values (200 replicates) and posterior probabilities indicated for each node (when superior to 50 and 0.5 respectively).
Species assignments for the six genetic groups
Shell characters traditionally used in Conus taxonomy were investigated in all the specimens analysed molecularly. The goal was to identify diagnostic characters in each of the six genetic groups that would allow an attribution of each of these groups to a species or subspecies name available in literature. To do so, we mainly refer to the name-bearing types of the relevant forms shown in Fig. 5, and the description of shell variation and map ranges provided by Röckel et al. (1995). Protoconchs in all species of the Conus orbignyi-complex are non-diagnostic; all are multispiral and indicate planktotrophic larval development. By contrast, teleoconchs differ by subtle but apparently stable differences:
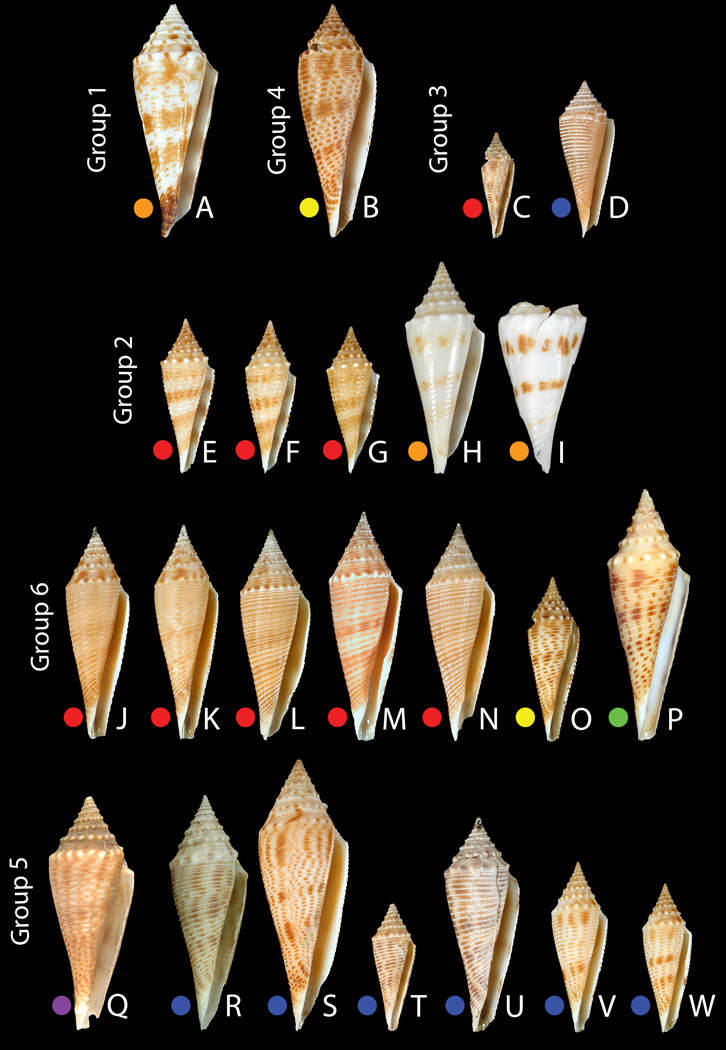
Group 1 - Conus comatosa. As shown in Fig. 6A, the identification of the Chesterfield specimen as C. comatosa seems unambiguous, based on its mostly smooth, rather than tuberculate, spiral shoulder and four spiral color bands, rather than three. All other members of the orbignyi complex included in this study have nodulose spiral shoulders, making C. comatosa conchologically distinctive. The dark brown color of the anterior end of the body whorl is another distinctive character absent in the other forms.
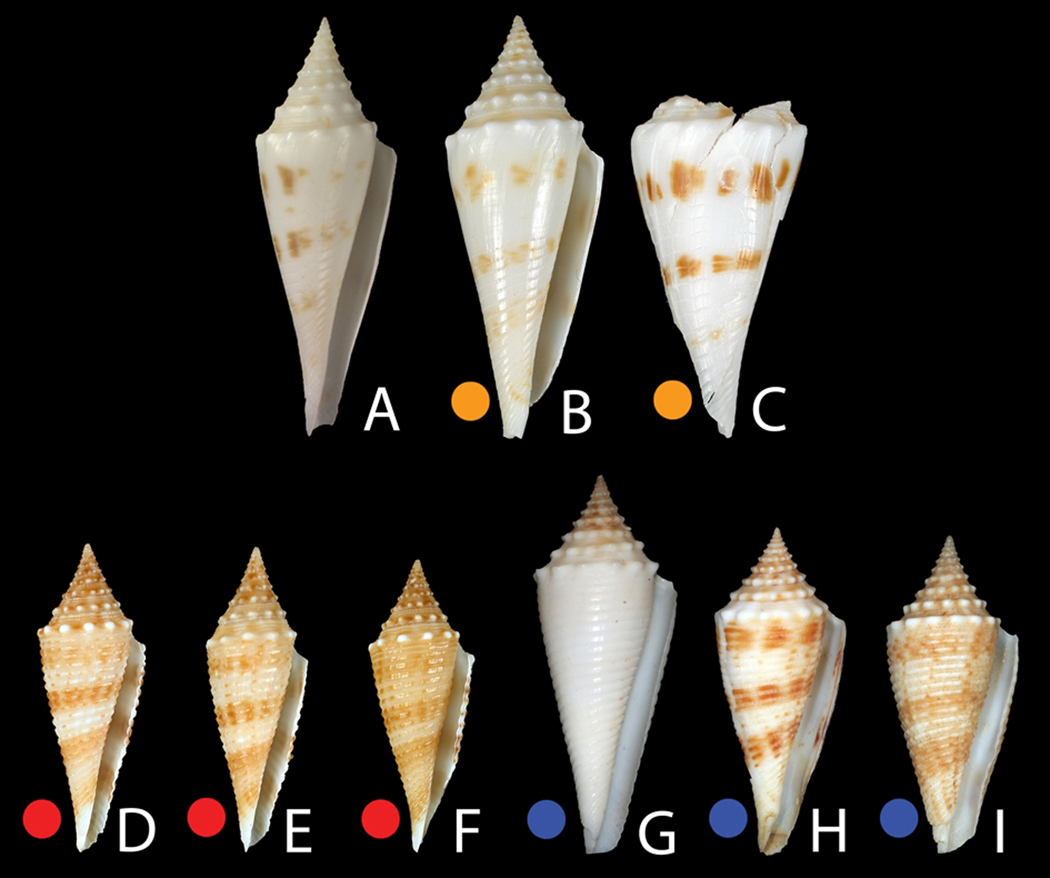
Group 2 - Conus coriolisi. The molecular data obtained were from specimens collected in Vanuatu and the Coral Sea; despite the close genetic distance using the various molecular markers in the two populations (<1%), they are unexpectedly morphologically divergent (see Fig. 6E–I and Fig. 7). The Chesterfield/Coral Sea material is similar to the holotype of C. orbignyi coriolisi (type locality: Capel Bank, Coral Sea) with weak to almost absent spiral ribbons and white ground color. The Chesterfield specimens, while somewhat broader in their shell outline than the type, have the characteristic interrupted brown blotches organized into three bands characterizing the type. The Vanuatu specimens belonging to C. coriolisi have stronger spiral sculpture and a pale tan ground color. The brown bands are both stronger and more continuous than in the Chesterfield specimens including the type. Given the genetic distance (~9%) of Group 2 specimens to C. orbignyi (Group 5) and the fact that C. comatosa is its sister species in both markers (Fig. 3) justify treating C. coriolisi as a distinct species and not a subspecies of C. orbignyi. It is notable that a very distinctive Philippine morph primarily collected from Aliguay Island and Panglao, but also represented in the Aurora 2007 material as empty shells (see below), is most similar to Group 2 specimens from Vanuatu based on overall shell shape, structure of the sutural ramp, degree of spiral sculpture, and ground color. No molecular data on such Philippine material has been obtained, but based on the similar morphology of the Philippine material to the Vanuatu specimens, and based on the molecular results, we tentatively assign the Philippines specimens (Table 2 – see description below) and the Group 2 specimens from Vanuatu to C. coriolisi, even though they are morphologically distinct from C. coriolisi from Coral Sea specimens including the type.
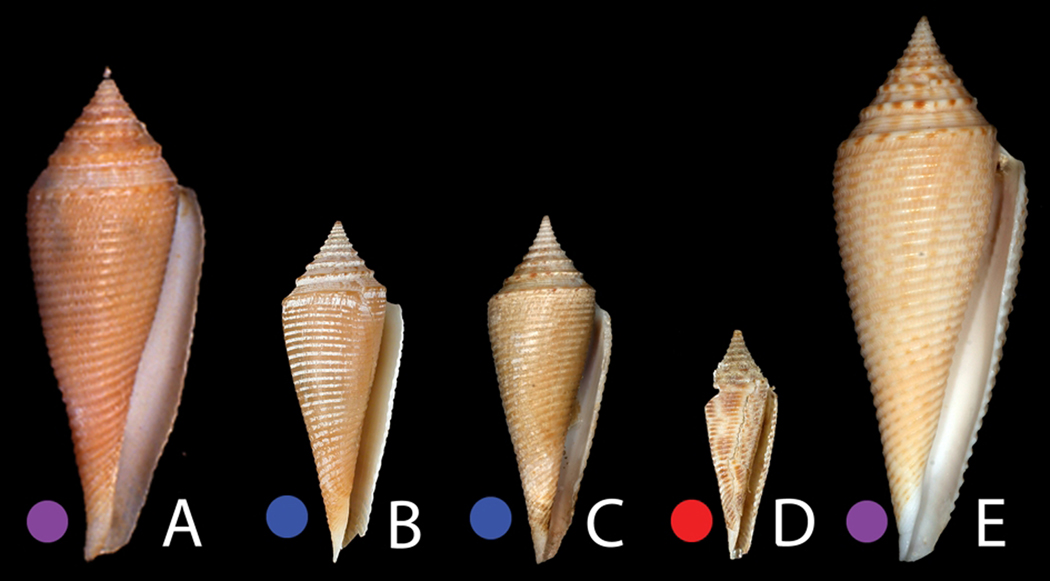
Group 3 - Conus pseudorbignyi. Two different specimens represent this group, one from Aurora in the Philippines and one from Vanuatu (Fig. 6C–D). The Aurora specimen, as shown in Fig. 8C, though smaller, is generally similar to the holotype of C. pseudorbignyi, which was collected in Taiwan. The Vanuatu specimen is quite divergent from both the holotype and the Philippine specimens in shell pattern, having much more distinctive brown blotches. Both the Philippine and Vanuatu material differ in their shell morphology from Taiwanese specimens collected in recent years (see Fig. 8E) and assigned by Röckel et al. (1995) to C. pseudorbignyi. These specimens have strong alternating brown blotches between the nodules on the prominent sutural ridge and are generally larger than the holotype and other specimens examined in this study. No molecular data are available for these Taiwanese specimens of C. pseudorbignyi to assess their genetic relationship to the Philippine and Vanuatu forms. We provisionally assign all of the specimens shown in Fig. 8 to C. pseudorbignyi, despite the divergence in shell morphology. A specimen figured by Wilson (1994) from deep water off Western Australia, and identified as Conus orbignyi, likely belongs to the Conus pseudorbignyi complex. This assignment would extend the distribution of Conus pseudorbignyi in the southern hemisphere over a much wider range than has previously reported, from Vanuatu to Port Hedland, Western Australia.
Group 4 - Conus joliveti. A single specimen from the Solomon Islands forms a discrete branch in both trees (Fig. 3 and 4). The taxonomic identity of this form merits discussion. The specimen in Fig. 6B appears morphologically closest to a specimen illustrated by Moolenbeek et al. (2008) from Fiji that these authors assigned to C. pseudorbignyi (see Plate 3, Fig. 24 of that paper). However, the specimen illustrated seems very different from C. pseudorbignyi, the holotype of which is from Taiwan, and is morphologically distinct from the specimen here figured from the Solomons. In the same paper, the authors describe a new species, C. joliveti. This form appears more closely related to the specimen labeled C. pseudorbignyi than does C. pseudorbignyi from Taiwan. It is clear that because only one specimen is available, more material - notably topotypical material from Fiji - needs to be analyzed before the identity of this species can be definitively established. We tentatively designate this branch as C. joliveti.
Group 5 - Conus orbignyi. The morphology and geographical location of specimens from both Taiwan and the Philippines are consistent in their nodulose sutural ridges with alternating brown blotches on the spire, their slender body whorl outline and the brown banding pattern with assigning these to C. orbignyi (Fig. 6Q–W). Within the Philippines, live specimens were analyzed from both Panglao Island, in the Central Philippines, and the Aurora 2007 expedition (Table 1).
Group 6 - Conus elokismenos. Three morphologically and geographically distinct classes of specimens are included in this group: a single specimen from Madagascar, similar to the name-bearing type of the subspecies C. o. elokismenos, a single specimen from the Solomons, and a majority of specimens from Vanuatu (see Fig. 6J–P). The specimens from Vanuatu are morphologically distinct from the Solomon Is and Madagascar specimens, being less nodulose, and with a more restrained pattern of brown markings in the body whorl, without the distinctive bold dashes on the ribbons characteristic of both the Solomon Is and Madagascar specimens. Furthermore, C. orbignyi is not reported from Western Australia (Röckel et al., 1995), and the discontinuous distribution of C. elokismenos is thus probably real. Together with the high geographic distances, particularly between Madagascar and the South Pacific, this would justify the separation of the various forms into subspecies, or even species. Tentatively, we designate the diverse morphological forms in this group as the “C. elokismenos complex”.
Fig. 5.
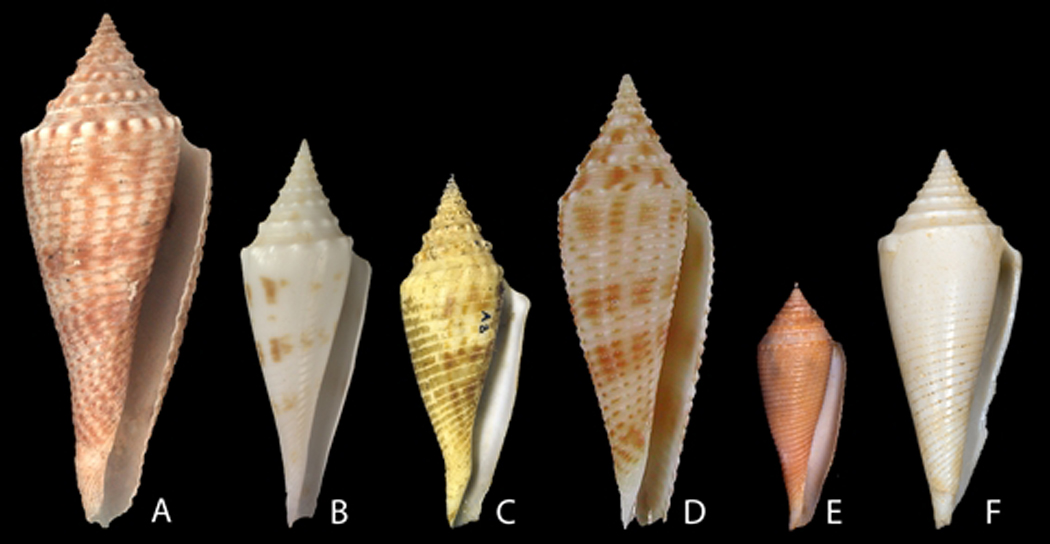
Illustration of type specimens (scaled). A: Conus orbignyi Audouin, 1831. Holotype MNHN 2532, H 53.2 mm. B: Conus orbignyi coriolisi Moolenbeck & Richard, 1995. Holotype MNHN 2570, H 41.5 mm. C: Conus orbignyi aratus Kilburn, 1973 (renamed C. o. elokismenos). Holotype NM, H 60 mm. D: Conus joliveti Moolenbeck, Röckel & Bouchet, 2008. Holotype MNHN 21036, H 29.1 mm. E: Conus pseudorbignyi Röckel & Lan, 1981. Holotype TMT. H 45.5 mm. F: Conus comatosa Pilsbry, 1904. Lectotype (Coomans et al., 1985) ANSP 85590, H 43.4 mm. Photos C. Reyens (A, B), A. Robin (D).
Fig. 6.
Specimens used for the molecular analyses (scaled). A: Group 1 – C. comatosa, Chesterfield, MNHN IM200730681 (46.8 mm). B: Group 4 – C. joliveti, Solomons, MNHN IM200730925 (46.9 mm). C–D: Group 3 – C. pseudorbignyi. C: Vanuatu, MNHN IM200730942 (21.2 mm). D: Philippines, MNHN IM200730899 (31.5 mm). E–I: Group 2 – C. coriolisi. E–G: Vanuatu. E: MNHN IM200730660 (31 mm). F: MNHN IM200730823 (30.7 mm). G: MNHN IM200730666 (29.4 mm). H-I: Chesterfield. H: MNHN IM200730836 (42.5 mm). I: MNHN IM200730842 (50 mm). J–P: Group 6 – C. elokismenos. J–N: Vanuatu. J: MNHN IM200730848 (42.5 mm). K: MNHN IM200730846 (43 mm). L: MNHN IM200730714 (42.4 mm). M: MNHN IM200730938 (45.9 mm). N: MNHN IM200730893 (43.6 mm). O: Solomons, MNHN IM200730939 (33 mm). P: Madagascar, MNHN IM20097513 (50.5 mm). Q–W: Group 5 – C. orbignyi. Q: Taiwan, FLMNH UF 327736 (47 mm). R–W: Philippines. R: MNHN IM200717921 (47.3 mm). S: MNHN IM200730813 (54.4 mm). T: MNHN IM200730729 (25.5 mm). U: MNHN IM200730773 (43 mm). V: MNHN IM200730785 (34.2 mm). W: MNHN IM200730815 (29.9 mm).
Fig. 7.
Specimens assigned to the C. coriolisi complex. A: Conus orbignyi coriolisi Moolenbeck & Richard, 1995. Holotype MNHN 2570, H 41.5 mm. B–C: Chesterfield. B: MNHN IM200730836 (42.5 mm). C: MNHN IM200730842 (50 mm). D–E: Vanuatu. D: MNHN IM200730660 (31 mm). E: MNHN IM200730823 (30.7 mm). F: MNHN IM200730666 (29.4 mm). G–I: Philippines. G: Aliguay island (38 mm). H: Aliguay Island (33 mm). I: Aurora 2007, station CP2666 (32 mm). Photos C. Reyens (A).
Fig. 8.
Specimens assigned to the C. pseudorbignyi complex. A: Conus pseudorbignyi Röckel & Lan, 1981. Holotype TMT. H: 45.5mm. B–C: Philippines. B: MNHN IM200730899 (31.5 mm). C: Aurora 2007, station CP2760 (32 mm). D: Vanuatu, MNHN IM200730942 (21.2 mm). E: Taiwan, Olivera collection, C. pseudorbignyi (50 mm).
Aurora 2007 empty shells: field collection data
A total of 110 adult shells were examined and, on the basis of shell morphology, were tentatively assigned to one of the classes in the C. orbignyi complex (Table 2). The allocation of juvenile shells to one or another form was too uncertain, and these are not listed in Table 2. Most of the specimens tentatively assigned to a specific taxon in Table 2 (N=93) correspond to the typical C. orbignyi form from the Philippines (Fig. 5A, Fig. 6R–W). These are strongly nodulose, with the characteristic darker brown bands on a body whorl and a lighter brown ground color. As explained before, several dead shells (12) presented the same morphology as the specimens collected in Vanuatu and are assigned to C. coriolisi (Fig. 5B, Fig. 6E–I). These have a broader body whorl, a more continuous banding pattern and a distinctly whitish ground color. Five other specimens were characterized by the typical morphology of C. pseudorbignyi from the Philippines (Fig. 5E). These were far less modulose, with finer spiral sculpture in the body whorl and the brown bands present in the other two forms were absent or obsolete.
All specimens attributed to C. orbignyi and C. coriolisi were found deeper than 150 meters. C. coriolisi always co-occurred in the same station as C. orbignyi. One specimen of C. pseudorbignyi was also collected together with C. orbignyi and C. coriolisi in a single station, but other specimens of C. pseudorbignyi were from stations shallower than 150 m in depth.
Discussion
Success and limits of the molecular approach
The combination of the results obtained with the mitochondrial COI gene and the nuclear ITS2 gene defines at least six distinctive genetic groups within the C. orbignyi complex. With both genes, each group is characterized by intra-group distances lower than intergroup distances, and the monophyly of each group is supported when multiple specimens were analyzed. It is thus reasonable to consider that each of these six genetic groups corresponds to a different species.
However, we cannot exclude the possibility that some of these groups are themselves species complexes comprising several species, in particular for Group 6. The genetic distance between the specimen collected in Madagascar (MNHN IM20097513) and the others is ~ 5–6%, and it could thus be considered as a different species (this exceeds the interspecific thresholds that have been reported for molluscs – Meyer & Paulay, 2005; Mikkelsen et al., 2007; Duda et al., 2008). The alternative hypothesis would be to consider Group 6 as an allopatrically structured superspecies complex, widely-distributed across the southern Indo-Pacific, with a considerable genetic distance between disjunct populations, and adjacent geographic populations still able to exchange genetic material (Knowlton, 2000; Klanten et al., 2007; Yu & Chu, 2006, but see Rhodes et al., 2003; Rocha, 2004; Meyer et al., 2005). The presence or absence of genetically-related populations in the intervening geographic areas is a key factor to be evaluated. At the present time, because of the depth range and scarcity of the species involved, the dataset is insufficient to address these hypotheses.
The paucity of the sampling is also certainly responsible for the profile of the histogram of COI distances. In our conservative approach, we considered that the second and third modes of the histogram (Fig. 2) correspond to intraspecific distances. In this case, increasing the sampling by including more specimens from different regions within each species could potentially merge the first three modes into a single one. As emphasized previously, especially in the DNA Barcoding literature, the quality of the sampling (both in terms of number of specimens and number of geographical areas sampled) is a key issue to accurately recover – or discover – species boundaries (Moritz & Cicero, 2004; Meyer & Paulay, 2005; Eckert et al., 2008; Monaghan et al., 2009). Although resulting from several years of biodiversity exploration by MNHN (Bouchet et al., 2008), our sampling still remains patchy. All the species analysed here live in deep water, and most of them are rare to uncommon. Delimiting species with such uneven sampling is currently one of the main challenges for the molecular taxonomist (Morando et al., 2003; Knowles & Carstens, 2007; Bouchet et al., 2009; Monaghan et al., 2009), but pending better methods to discriminate between the two alternative hypotheses “one broad species with divergent populations” and “several allopatric species” a conservative approach as applied here seems to be the most prudent course.
Another limitation of our results, not linked to the quality of the sampling but to the taxa analysed, is the lack of resolution using the ITS2 gene. The monophyly of Group 5 is not established using this gene, as Group 6 is included in paraphyly, even if several mutations and one indel clearly separate the two groups. Incomplete lineage sorting can explain this pattern (Maddison & Knowles, 2006; Elejalde et al., 2008), and this could be tested by analyzing a more variable nuclear gene. However, the Conus genome remains poorly known, and apart from the classic nuclear genes (e.g. 28S, H3, 18S), used to resolve deeper phylogenetic relationships (e.g. Colgan et al., 2003, 2007; Puillandre et al., 2008) and thus less variable than the ITS2 gene at the species level, only conotoxins have been used in a similar study (Duda et al., 2008). We tested the conotoxin primers used by Duda et al. (2008) in the orbignyi complex, but because of the high divergence between the complex they studied (C. sponsalis, included in the large major clade - Duda & Kohn, 2005) and the orbignyi complex (included in the small major clade), no amplicons were obtained. Amplifying conotoxin genes from genomic DNA in the small major clade will require developing a new set of primers.
A revised taxonomy for the Conus orbignyi complex
By carrying out a combination of a molecular phylogenetic analysis of all available specimens and a morphological survey of material collected at a single site (off Aurora, Luzon Island in the Philippines) and more generally within the Philippines, we provide taxonomic resolution within the C. orbignyi complex and gain insights into the biogeography of the putative species. One surprising discovery is that C. comatosa is a nested member within the complex. This phylogenetic position implies that the smooth sutural shoulder that differentiates this species from the others is derived from a nodulose plesiomorphic state. Specimens in the C. orbignyi clade analyzed in this study would have been included in four different species based on shell characters, C. orbignyi, C. pseudorbignyi, C. joliveti and C. comatosa (Röckel et al., 1995; Moolenbeek et al., 2008). C. orbignyi was believed to have a discontinuous distribution, with three potential subspecies, C. o. orbignyi, C. o. elokismenos and C. o. coriolisi. Based on the molecular results, we elevate the three subspecies to species rank (C. orbignyi, C. elokismenos and C. coriolisi), two of which coexist in the SW Pacific. Each of these six taxa is discussed in turn.
Two species included in the redefined orbignyi complex are represented in the molecular dataset by a single specimen: C. comatosa and C. joliveti. The recently named Conus joliveti has been described from material collected in Fiji, and whether or not it is molecularly similar to specimen MNHN IM200730925 identified as C. joliveti remains to be assessed. Conus comatosa is a well-known species, which, unlike other forms in the clade, does not appear to show great geographic variation. The Chesterfield specimen analyzed herein is virtually identical to specimens of Conus comatosa from the Philippines. Unfortunately, no live specimens of C. comatosa from the Philippines were available to include in the molecular analysis.
Specimen MNHN IM200730899 (Fig. 6D) matches the illustration of C. pseudorbignyi from the Philippines by Röckel et al. (1995). However, specimen MNHN IM200730942 from Vanuatu (Fig. 6C), placed in the same genetic group and thus putatively in the same species, seems to have shell characters divergent from Conus pseudorbignyi from the type locality Taiwan, and a number of distinctive morphological differences from the Philippine specimens as well (see Fig. 5E). Geographic variation was already apparent in the illustration of Conus pseudorbignyi in Röckel et al. (1995 - Plate 56); the Taiwanese specimens are distinctive from both the Philippine specimen as well as from the specimen from Sulawesi. Molecular analysis of Conus pseudorbignyi from Taiwan (the type locality) is clearly desirable to confirm our assignment of these specimens to Conus pseudorbignyi. Thus, there appears to be morphological divergence between C. pseudorbignyi specimens from Taiwan, Aurora and Vanuatu.
We regard C. orbignyi as a rather variable species (Fig. 5A and 6Q–W), and the results of the Aurora expedition suggest that at this site, this species is only found in water deeper than 150 meters (Table 2). It is likely to be present at a variety of Philippine sites, but is not common in commercially available material; thus the recent book on Philippine molluscs by Poppe (2008) does not illustrate any true C. orbignyi as defined by the molecular phylogeny. A typical specimen of C. orbignyi from the Philippines has been illustrated earlier by Springsteen & Leobrera (1986) and variation in typical Conus orbignyi was illustrated by Röckel et al. (1995 - Plate 56, specimens 2–4 from Taiwan and the Philippines). None of the specimens from Southern Hemisphere localities were found to belong to this species, suggesting that C. orbignyi is a Northwestern Pacific species, found from the Philippines to Japan. During the Aurora 2007 expedition, this was one of the most frequently collected species in the Conidae.
Surprisingly distant from C. orbignyi on the basis of molecular phylogeny is the form previously called C. orbignyi coriolisi. We suggest that this is not only a distinct species from C. orbignyi, but one that is morphologically variable as well (Fig. 6E–I). At the present time, specimens assigned to Conus coriolisi occur in the Western Pacific from Fiji to Queensland, and north to the Central Philippines. Our assignment of Philippine specimens to this species needs to be verified by molecular data. However, these specimens are clearly distinct from the typical C. orbignyi and have a much closer morphological similarity to specimens of C. coriolisi from Vanuatu (Fig. 5A–B and 6E–I). Two of the specimens assigned to C. orbignyi by Poppe (2008: Plate 641, Fig. 7a and 7b) as well as one of the specimens illustrated by Springsteen & Leobrera (1986: Plate 71, Fig. 13, rightmost specimen) appear to be C. coriolisi. The specimen illustrated in Röckel et al. (1995) as C. coriolisi is a typical Coral Sea specimen, morphologically similar to the Chesterfield specimens analyzed in this study. However, these authors also illustrate a specimen from Queensland assigned to C. orbignyi that is similar to C. coriolisi from Vanuatu. Thus, C. coriolisi appears to be a distinctive species with consistent morphological variation that overlaps with C. orbignyi in its geographic range in the Philippines. Based on the specimens examined herein, Chesterfield individuals possess a white ground color, instead of light brown; and the brown markings are largely restricted to three bands. A distinctive form from Aliguay Island, Philippines, tentatively assigned to C. coriolisi, is pure white and highly nodulose; the shell morphology seems most closely related to C. coriolisi (see Fig. 5B) than to any other species in the clade, but molecular data may reveal that this is a distinct form.
Conus elokismenos is now elevated to species status. Formerly, regarded as a Southeast African/Madagascar form, molecular data indicate that specimens in the Solomon Islands and Vanuatu are genetically allied to the Madagascar material. Thus, while Conus orbignyi is the Northern Hemisphere species in the complex, the morphologically similar Conus elokismenos clade exists in the Southern Hemisphere. As circumscribed herein, there may be a justification for dividing Conus elokismenos into different subspecies or even different species (as discussed above), turning the C. elokismenos clade into a species complex itself. Further sampling would be necessary to justify this step.
Conclusion
The analysis carried out in this study on a clade of cone snails highlights why the taxonomic assignment at the species level based purely on morphology has been extraordinarily challenging for many hyper-diverse lineages. There were four previously recognized species in the complex we have analyzed, but we have demonstrated that additional species should be recognized. Some of these species are distributed over a broad geographic area; whereas others appear more geographically restricted. More unexpectedly, the results show that some taxa appear to exhibit a larger geographical morphological variation than others, whereas mode of larval development – and thus inferred dispersal capacity – is the same (planktotrophic) throughout the species complex. However, we refrain from speculating too much on this issue because (a) our specimen sampling is still rather thin, and uneven across the terminal taxa involved (e.g. a single specimen of C. joliveti vs seven specimens of C. orbignyi), and (b) some of the taxa here recognized as species (elokismenos) may themselves be species complexes.
A useful approach to understanding a complex such as the one analyzed in this study is to focus on the morphologically divergent forms found in a single locality, such as was provided by the material from the Aurora 2007 Expedition. At this locality, three clearly separable morphospecies could be unambiguously differentiated (see Fig. 5A, B and E). Two of these were recovered alive, and as expected, they showed a genetic divergence that corresponded to the morphological divergence at this locality. Dead shells from a third distinct morphospecies were recovered, but lacked tissue for genetic testing. All three forms found at Aurora also occur at other localities, and to different degrees, they vary geographically. This intraspecific divergence in morphology as a function of geography in species complexes that are morphologically similar confounds the taxonomy based on morphology alone. In order to accurately provide a taxonomy for hyper-diverse groups, understanding inter-regional morphologic variation in the context of molecular data is highly desirable. Potentially, the situation can be resolved through an intensive analysis of a few geographically-separated localities where the species complex occurs. Thus, expeditions such as Aurora in 2007 and Santo in 2006 provide the type of material that can help elucidate species complexes in hyper-diverse groups such as the Conidae and beyond.
Acknowledgments
This work was supported in part by Program Grant GM48677 from the US National Institute of General Medical Sciences (to BMO) and by ICBG grant 1U01TW008163 from the Fogarty Center, NIH, Margo Haygood, Principal Investigator. Key material originates from the following expeditions. (A) Philippines. The PANGLAO 2005 cruise on board M/V DA-BFAR associated the USC, MNHN (co-PI Philippe Bouchet) and the Philippines Bureau of Fisheries and Aquatic Research (BFAR; co-PI Ludivina Labe). The AURORA 2007 cruise also on board M/V DA-BFAR associated the National Museum of the Philippines (NMP, co-PI Marivene Manuel), MNHN (co-PI Philippe Bouchet) and BFAR, and was made possible through a grant from the Lounsbery Foundation. (B) Vanuatu. Material originates from the MNHN-IRD-PNI Santo 2006 expedition, which was made possible by grants, among others, from the Total Foundation and the Niarchos Foundation. (C) Coral Sea and Solomon Islands. The EBISCO and SALOMON cruises took place on board R/V Alis deployed from Nouméa by the Institut de Recherche pour le Développement (IRD). Bertrand Richer de Forges was cruise leader for the Solomons, Coral Sea and Vanuatu expeditions. Ellen Strong and Yuri Kantor are thanked for their role in molecular sampling during these expeditions. The authors thank Virginie Héros for assistance in monitoring Aurora 2007 material and Hans-Jörg Niederhöfer (Staatliches Museum für Naturkunde Stuttgart), Virginie Héros (Muséum National d’Histoire Naturelle, Paris) and Paul Callomon (Academy of Natural Sciences, Philadelphia) for their help in providing pictures of type specimens.
References
- Appleton RD, Palmer RA. Water-borne stimuli released by predatory crabs and damaged prey induce more predator-resistant shells in a marine gastropod. Proceedings of the National Academy of Sciences. 1988;85:4387–4391. doi: 10.1073/pnas.85.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchet P, Héros V, Lozouet P, Maestrati P. A quater-century of deep-sea malacological exploration in the South and West Pacific: Where do we stand? How far to go? In: Héros V, Corwie RH, Bouchet P, editors. Tropical deep-sea benthos 25. Mémoires du muséum national d’histoire naturelle 196: 9–40. 2008. Paris ISBN: 978-2-85653-614-8. [Google Scholar]
- Bouchet P, Lozouet P, Sysoev AV. An inordinate fondness for turrids. Deep-Sea Research II. 2009;56:1724–1731. [Google Scholar]
- Colgan DJ, Ponder WF, Beacham E, Macaranas J. Gastropod phylogeny based on six fragments from four genes representing coding or non-coding and mitochondrial or nuclear DNA. Molluscan Research. 2003;23:123–148. [Google Scholar]
- Colgan DJ, Ponder WF, Beacham E, Macaranas J. Molecular phylogenetics of Caenogastropoda (gastropoda: Mollusca) Molecular Phylogenetics and Evolution. 2007;42:717–737. doi: 10.1016/j.ympev.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Coomans HE, Moolenbeek RG, Wils E. Alphabetical revision of the (sub)species in recent conidae 9 Ebraeus to extraordinarius with the description of conus elegans ramalhoi, nov. subspecies. Basteria. 1985;50:93–150.. [Google Scholar]
- De Queiroz K. The general lineage concept of species, species criteria, and the process of speciation: A conceptual unification and terminological recommendations. In: Howard DJ, Berlocher SH, editors. Endless forms: Species and speciation. Oxford: Oxford University Press; 1998. pp. 57–75. [Google Scholar]
- De Queiroz K. Species concepts and species delimitation. Systematic Biology. 2007;56:879–886. doi: 10.1080/10635150701701083. [DOI] [PubMed] [Google Scholar]
- Duda TF, Bolin MB, Meyer C, Kohn AJ. Hidden diversity in a hyperdiverse gastropod genus: Discovery of previously unidentified members of a Conus species complex. Molecular Phylogenetics and Evolution. 2008;49:867–876. doi: 10.1016/j.ympev.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Duda TF, Kohn AJ. Species-level phylogeography and evolutionary history of the hyperdiverse marine gastropod genus Conus. Molecular Phylogenetics and Evolution. 2005;34:257–272. doi: 10.1016/j.ympev.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Eckert CG, Samis KE, Lougheed SC. Genetic variation across species’ geographical ranges: The central–marginal hypothesis and beyond. Molecular Ecology. 2008;17:1170–1188. doi: 10.1111/j.1365-294X.2007.03659.x. [DOI] [PubMed] [Google Scholar]
- Edwards SV. Is a new and general theory of molecular systematics emerging? Evolution. 2009;63:1–19. doi: 10.1111/j.1558-5646.2008.00549.x. [DOI] [PubMed] [Google Scholar]
- Elejalde MA, Madeira MJ, Arrébola JR, Munoz B, Gomez-Moliner BJ. Molecular phylogeny, taxonomy and evolution of the land snail genus Iberus (pulmonata: Helicidae) Journal of Zoological Systematics & Evolutionary Research. 2008:193–202. [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology. 1994;3:294–299. [PubMed] [Google Scholar]
- Funk DJ, Omland KE. Species-level paraphyly and polyphyly: Frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annual Review of Ecology Evolution and Systematics. 2003;34:397–423. [Google Scholar]
- Gaines CA, Hare MP, Beck SE, Rosenbaum HC. Nuclear markers confirm taxonomic status and relationships among highly endangered and closely related right whale species. Proceedings of the Royal Society B. 2005;272:533–542. doi: 10.1098/rspb.2004.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/nt. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hebert PDN, Stoeckle MY, Zemlak TS, Francis CM. Identification of birds through DNA barcodes. Plos Biology. 2004;2:1657–1663. doi: 10.1371/journal.pbio.0020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F, Hall B. MrBayes: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Keane TM, Creevey CJ, Pentony MM, Naughton TJ, McInerney JO. Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evolutionary Biology. 2006;6:1–17. doi: 10.1186/1471-2148-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klanten SO, Choat JH, van Herwerden L. Extreme genetic diversity and temporal rather than spatial partitioning in a widely distributed coral reef fish. Marine Biology, 2007;150:659–670. [Google Scholar]
- Knowles LL, Carstens BC. Delimiting species without monophyletic gene trees. Systematic Biology. 2007;56:887–895. doi: 10.1080/10635150701701091. [DOI] [PubMed] [Google Scholar]
- Knowlton N. Molecular genetic analyses of species boundaries in the sea. Hydrobiologia. 2000;420:73–90. [Google Scholar]
- Kumar S, Tamura K, Nei M. Mega3: Integrated software for molecular evolutionary analysis and sequence alignment. Briefings in Bioinformatics. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Linnen CR, Farrell BD. Phylogenetic analysis of nuclear and mitochondrial genes reveals evolutionary relationships and mitochondrial introgression in the sertifer species group of the genus Neodiprion (hymenoptera: Diprionidae) Molecular Phylogenetics and Evolution. 2008;48:240–257. doi: 10.1016/j.ympev.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Knowles LL. Inferring phylogeny despite incomplete lineage sorting. Systematic Biology. 2006;55:21–30. doi: 10.1080/10635150500354928. [DOI] [PubMed] [Google Scholar]
- Meyer CP, Geller JB, Paulay G. Fine scale endemism on coral reefs: Archipelagic differentiation in turbinid gastropods. Evolution. 2005;59:113–125. [PubMed] [Google Scholar]
- Meyer CP, Paulay G. DNA barcoding: Error rates based on comprehensive sampling. Plos Biology. 2005;3:1–10. doi: 10.1371/journal.pbio.0030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen N, Schander C, Willassen E. Local scale DNA barcoding of bivalves (mollusca): A case study. Zoologica Scripta. 2007;36:455–463. [Google Scholar]
- Monaghan MT, Wild R, Elliot, Fujisawa T, Balke M, Inward DJG, et al. Accelerated species inventory on Madagascar using coalescent-based models of species delineation. Systematic Biology. 2009;58:298–311. doi: 10.1093/sysbio/syp027. [DOI] [PubMed] [Google Scholar]
- Moolenbeek RG, Rockel D, Bouchet P. New records and new species of cones from deeper water off Fiji (Mollusca, Gastropoda, Conidae) Vita Malacologica. 2008;6:35–49. [Google Scholar]
- Morando M, Avila LA, Sites JW. Sampling strategies for delimiting species: Genes, individuals, and populations in the Liolaemus elongatus-kriegi complex (Squamata: Liolaemidae) in andean–patagonian South America. Systematic Biology. 2003;52:159–185. doi: 10.1080/10635150390192717. [DOI] [PubMed] [Google Scholar]
- Moritz C, Cicero C. DNA barcodes: Promise and pitfalls. Plos Biology. 2004;2:1529–1531. doi: 10.1371/journal.pbio.0020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HH, Corneli PS, Watkins M, Olivera B, Bandyopadhyay P. Multiple genes elucidate the evolution of venomous snail-hunting Conus species. Molecular Phylogenetics and Evolution. 2009;53:645–652. doi: 10.1016/j.ympev.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Olivera BM. Conus peptides: Biodiversity-based discovery and exogenomics. Journal of Biological Chemistry. 2006;281:31173–31177. doi: 10.1074/jbc.R600020200. [DOI] [PubMed] [Google Scholar]
- Palumbi S. Nucleic acids ii: The polymerase chain reaction. In: Hillis D, Moritz C, Mable BK, editors. Molecular systematics. Sunderland (MA): Sinauer Associates; 1996. pp. 205–247. [Google Scholar]
- Petit R, Excoffier L. Gene flow and species delimitation. Trends in Ecology and Evolution. 2009;24:386–393. doi: 10.1016/j.tree.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Poppe GT. Philippine marine mollusks volume ii. Conchbooks; 2008. [Google Scholar]
- Puillandre N, Baylac M, Boisselier MC, Cruaud C, Samadi S. An integrative approach of species delimitation in the genus Benthomangelia (Mollusca: Conoidea) Biological Journal of the Linnean Society. 2009;96:696–708. [Google Scholar]
- Puillandre N, Samadi S, Boisselier MC, Sysoev AV, Kantor YI, Cruaud C, et al. Starting to unravel the toxoglossan knot: Molecular phylogeny of the “turrids” (Neogastropoda: Conoidea) Molecular Phylogenetics and Evolution. 2008;47:1122–1134. doi: 10.1016/j.ympev.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Puillandre N, Sysoev A, Olivera BM, Couloux A, Bouchet P. Loss of planktotrophy and speciation: Geographical fragmentation in the deep-water gastropod genus Bathytoma (gastropoda, conoidea) in the western pacific. Systematics and Biodiversity, iFirst. 2010 [Google Scholar]
- Author. Tracer v1.4. 2007 Available from http://beast.bio.ed.ac.uk/Tracer. [Google Scholar]
- Rhodes KL, Lewis RI, Chapman RW, Sadovy Y. Genetic structure of camouflage grouper, Epinephelus polyphekadion (pisces: Serranidae), in the western central pacific. Marine Biology. 2003;142:771–776. [Google Scholar]
- Rocha LA. Mitochondrial DNA and color pattern variation in three western atlantic Halichoeres (Labridae), with the revalidation of two species. Copeia. 2004;4:770–782. [Google Scholar]
- Röckel D, Korn W, Kohn AJ. Manual of the living Conidae. Vol. I, indo-pacific. Wiesbaden: Christa Hemmen Verlag; 1995. [Google Scholar]
- Samadi S, Barberousse A. The tree, the network, and the species. Biological Journal of the Linnean Society. 2006;89:509–521. [Google Scholar]
- Simon C, Franke A, Martin A. The polymerase chain reaction: DNA extraction and amplification. In: Hewitt GM, Johnson WB, Young JPW, editors. Molecular techniques in taxonomy. New York: Springer-Verlag; 1991. pp. 329–355. [Google Scholar]
- Springsteen FJ, Leobrera FM. Shells of the philippines. Carfel Seashell Museum; 1986. [Google Scholar]
- Stamatakis A. Raxml-vi-hpc: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Terlau H, Olivera BM. Conus venoms: A rich source of novel ion channel-targeted peptides. Physiological Review. 2004;84:41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- Tucker JK, Tenorio MJ. Systematic classification of recent and fossil conoidean gastropods. Hackenheim, Germany: Conchbooks; 2009. [Google Scholar]
- Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PDN. DNA barcoding Australia’s fish species. Philosophical Transactions of the Royal Society B. 2005;360:1847–1857. doi: 10.1098/rstb.2005.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler QD. The new taxonomy. Boca Ratan: CRC Press; 2009. [Google Scholar]
- Wiens JJ. Species delimitation: New approaches for discovering diversity. Systematic Biology. 2007;56:875–878. doi: 10.1080/10635150701748506. [DOI] [PubMed] [Google Scholar]
- Wilson B. Australian marine shells. Vol. 2 prosobranch gastropods. Part two (neogastropods) Kallaroo, Western Australia: Odyssey Publishing; 1994. [Google Scholar]
- Yu DH, Chu KH. Low genetic differentiation among widely separated populations of the pearl oyster Pinctada fucata as revealed by aflp. Journal of Experimental Marine Biology and Ecology. 2006;333:140–146. [Google Scholar]