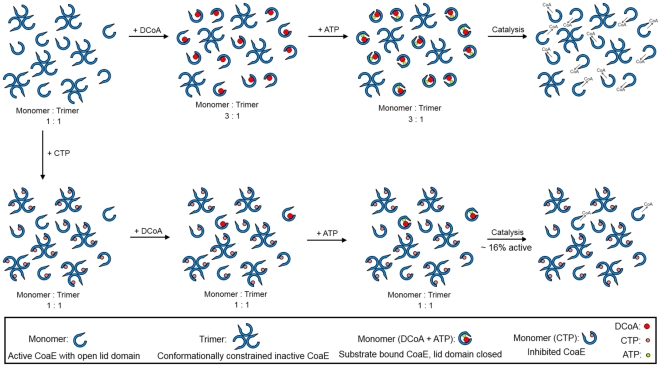
Figure 7. Schematic depicting the dynamic interplay between the enzyme's leading substrate, DCoA and the cellular metabolite, CTP, in regulating the activity of the last enzyme of mycobacterial CoA biosynthesis.
The enzyme is sequestered in a trimeric state that renders it inactive, potentially due to spatial constraints imposed on the mobile lid and CoA domains. DCoA, upon binding, induces monomerization, releasing these constraints and inducing conformational changes in the enzyme, allowing the phosphate donor, ATP, to bind and catalysis to occur. CTP, on the other hand, prevents DCoA from binding the enzyme by virtue of occupying a similar binding site on the enzyme, thereby preventing the oligomeric re-equilibration, reflected in a mere 16% active CoaE in the presence of 1 mM CTP. Thus the mycobacterial cell employs regulation at the last enzyme of CoA biosynthesis via two co-acting mechanisms.