Fig. 1.
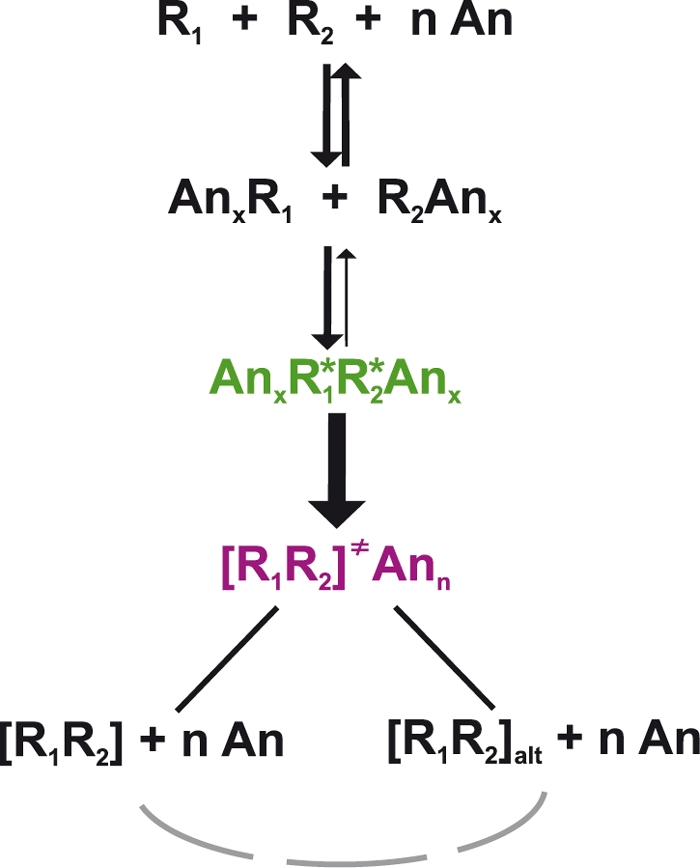
A generalized model for proteins that accelerate annealing and proteins capable of strand displacement (RNA chaperones). (A) In the RNA-only scenario, two complementary RNAs (R1 and R2) form an encounter complex and (once the necessary activation energy is reached and molecules show a favorable conformation and orientation) proceed to a transition state before they establish the RNA duplex. Apart from the thermodynamically favored duplex, alternative double strands (alt) can form. (B) Each RNA molecule is coated by several molecules of an annealer protein (An). The annealer protein supports the reaction by altering the structure of RNA molecules, which leads to annealing-competent RNA conformations. Thus, the fraction of encounter complexes that fall apart is decreased, and more encounters lead to successful procession to the transition state and, finally, the double strand. If the annealer protein has also strand displacement (SD) activity, it can reopen alternative structures, so that, eventually, only the thermodynamically favored duplex is found. (C) RNA duplexes that exceed a certain minimum stability will not disintegrate spontaneously. However, proteins with strand displacement activity destabilize such double strands by partially opening the duplex ends (indicated by parentheses). This allows an invading RNA, R3, to compete with R2 for base pairing with R1.
