FIGURE 2.
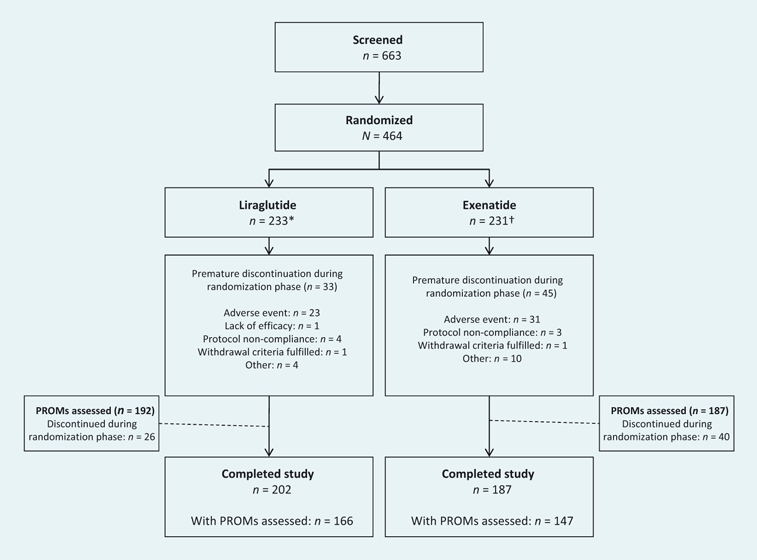
Patient disposition from screening until the end of the randomization phase (26 weeks) in Liraglutide Effect and Action in Diabetes 6 (LEAD 6). *Intention-to-treat (ITT), per-protocol (PP) and safety populations in the liraglutide treatment group were 233, 193 and 235, respectively. †ITT, PP and safety populations in the exenatide treatment group were 231, 172 and 232, respectively. PROMs, patient-reported outcome measures.
