Abstract
Helminths are known to elicit a wide range of immunomodulation characterized by dominant Th2-type immune responses. Our group previously showed that a DNA vaccine encoding the mycobacterial 65-kDa heat shock protein (DNA-hsp65) showed immunomodulatory properties. We also showed, using a helminth-tuberculosis (TB) co-infection model, that the DNA-hsp65 vaccine protected mice against TB. We next investigated the mechanistic role of the vaccine during helminth-TB co-infection. Clinically, helminth infection causes type 2 granulomas in the lung. Mice were immunized with DNA-hsp65 while they were submitted to the type 2 granuloma induction protocol by Schistosoma mansoni eggs infusion. In this work we investigated the effects of DNA-hsp65 on the pathology and immune response during the development of type 2 granuloma induced by S. mansoni eggs. Histologic analyses of lung parenchyma showed that the DNA-hsp65 vaccine protected mice against exacerbated fibrosis induced by Schistosoma eggs, and decreased the size of the granulomas. These changes were correlated with a reduction in the number of T cells specific for the egg antigens in the lung and also with modulation of Th2 cytokine expression. Taken together, our results showed that the adjuvant properties of the DNA-hsp65 vaccine regulated the immune response in this Th2 model, and resulted in a preserved lung parenchyma.
Helminth infections are widespread in the tropics, and are known to elicit a wide range of immunomodulation effects characterized by dominant T helper cell (Th) 2-type immune responses, chronic immune activation, as well as up-regulated regulatory T-cell activity. Our group previously showed that a DNA vaccine encoding the mycobacterial 65-kDa heat shock protein (DNA-hsp65), that protected mice and guinea pigs against tuberculosis (TB),1–3 also showed immunomodulatory properties in other diseases.4–7 We also showed that this DNA-hsp65 vaccine protected mice against TB in a helminth-TB co-infection model.8 These results led us to investigate the mechanistic role of the vaccine during helminth-TB co-infection.
One of the clinical manifestations of helminth infection is the presence of type 2 granulomas. The lung is the primary site of organ involvement in a range of granulomatous conditions.9 The immune response to Schistosoma mansoni eggs in mice results in the development of hepatic, intestinal, and pulmonary granulomas that lead to extensive fibrosis.10,11 The cellular composition of the granulomas includes eosinophils, alternatively activated (M2) macrophages, lymphocytes, neutrophils, mast cells, and fibroblasts.12 Further, the recruitment and migration of these cells into the site of inflammation are controlled by cytokines and chemokines. The complex cytokine–chemokine regulatory network has been well established, and dictates the profile of local chemokine expression during T-cell–mediated type 2 lung granuloma formation as a result of S. mansoni egg injection.13,14 To induce granulomas we used a system based on the embolization of S. mansoni eggs to the lungs. This approach leads the release of glycoproteins, referred to as S. mansoni soluble egg antigen (SEA), inducing a strong polarization of the immune response characterized by the production of Th2-related cytokines.15
Granuloma induction is mediated by major histocompatibility complex class II–restricted CD4+ T lymphocytes with specificities directed against egg antigens,16 and can occur in environments dominated by either Th1- or Th2-type cytokines.17,18 SEA promotes a dominant Th2 response, which involves the recruitment and activation of eosinophils, M2 macrophages, dendritic cells, and CD4+ Th2 cells.19 Although the egg-induced Th2 granulomatous response is required for host survival, the Th2 response is highly tissue destructive, partly because of the fibrotic scarring around these granulomas.15
One of the trademarks of granuloma development is the fibrosis progression in the area surrounding the inflammatory foci. Myofibroblast recruitment to the granuloma is a key effector cell in fibrogenesis. These myofibroblasts share features with smooth muscle cells because both can contract and contain α-smooth muscle actin (SMA) stress fibers. Myofibroblasts constitute the fibrotic foci and are the primary cell type responsible for the synthesis and deposition of extracellular matrix in the lung and thus are responsible for the structural remodeling that leads to the loss of alveolar function.20 During wound healing, the removal of inflammatory cells, including α-SMA+ myofibroblasts, is essential in preventing collagen deposition. Thus, α-SMA+ expression in lung tissue can be used as a marker of collagen-producing cells.
In this work we investigated the effects of the DNA-hsp65 vaccine on the immune response during the development of a type 2 granuloma and the consequences of these changes on lung tissue. Here, we show the protective properties of DNA-hsp65 vaccine against fibrosis induced by Schistosoma eggs, with decreases in both the granuloma size as well as in the fibrotic tissue surrounding the entrapped eggs. Importantly, the size of granulomas and the tissue commitment to fibrosis can determine the severity of disease as observed in lung sarcoidosis, in which the constriction of the bronchial wall as a result of these processes results in severe airway obstruction.21 The histologic changes observed after DNA vaccine administration were correlated with T-cell number reduction specific for the egg antigens in the lung, modulation of Th2 cytokines, and alternative macrophage markers. Together, these results provide a novel strategy to modulate the immune response and pathology during type 2 granuloma induction.
Materials and Methods
Animals
C57Bl/6 mice were purchased from Taconic (Germantown, NY). All mice were maintained under specific pathogen-free conditions and provided with food and water ad libitum in the Unit for Laboratory Animal Medicine facility at the University of Michigan Medical School. All animal protocols were approved by the University Laboratory Animal Medicine.
Plasmid Derivation
The pVAX1hsp65 construction22 and pVAX1 vector were prepared using the Endo-Free Plasmid Giga kit (Qiagen, Hildenberg, Germany). The endo-free condition of plasmids was determined by the Limulus Amebocyte Lysate test as recommended by European and US Pharmacopoeias,23 using the QCL 1000 Limulus Amebocyte Lysate test kit (Cambrex, Walkersville, MD).
Immunization Procedures and Granuloma Induction
Immunization was performed using five animals per group. Fourteen days after intraperitoneal injection of 3000 S. mansoni eggs, the plasmid containing the DNA insert (DNA-hsp65) was administered by intramuscular injection (50 μg in 50 μL of 25% buffered sucrose) into each quadriceps muscle three times at 1-week intervals (total dose, 300 μg of plasmid DNA). Seven days after the last DNA vaccine dose, mice received another 3000 S. mansoni eggs by intravenous route. Eight days after this injection we analyzed the histopathology and host immune response.
Protein Analysis of Cytokines
Murine cytokine levels were measured in 50-μL samples from whole-lung homogenates using a Bio-Plex bead-based cytokine assay purchased from Bio-Rad (Hercules, CA). The cytokine levels in lung homogenates were normalized to total protein levels (in milligrams) present in cell-free preparations of each sample measured by the Bradford assay, as described previously.24
Histologic and Immunofluorescent Analyses
Individual excised lung lobes were inflated and fixed with 10% buffered formalin and stained with H&E and Masson's Trichrome as previously described.25 The areas of the granulomas were measured in a blinded fashion on H&E-stained sections of paraffin-embedded lungs using computer-assisted morphometry as previously described.24,25 A minimum of 20 granulomas per lung section were measured.
For immunofluorescent analysis, lungs were embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA) and then frozen in liquid nitrogen. Seven-micron cryostat sections were fixed in ice-cold acetone, then incubated with primary antibodies followed by appropriate Alexa-labeled secondary antibodies (Invitrogen Corporation, Carlsbad, CA). Finally, the sections were analyzed by the Zeiss LSM 510 Confocal Microscope System (Carl Zeiss, Inc., Thornwood, NY).
Reverse Transcription and Real-Time Quantitative PCR Analysis
Total RNA was isolated from whole lungs or cultured cells using TRIzol (Invitrogen) according to the manufacturer's instructions. In brief, a total of 2.0 μg of RNA was reverse transcribed to yield cDNA in a 25-μL reaction mixture containing 1× first-strand (Life Technologies; Invitrogen), 250 ng oligo-dT (deoxy-thymine nucleotides) primer, 1.6 mmol/L deoxynucleoside triphosphates (dNTPs) (Invitrogen), 5 U RNase inhibitor (Invitrogen), and 100 U of Moloney murine leukemia virus reverse transcriptase (Invitrogen) at 38°C for 60 minutes. The reaction was stopped by incubating the cDNA at 94°C for 10 minutes. Real-time quantitative PCR analysis was performed by using the ABI 7700 Sequence Detector System (PE Applied Biosystems, Foster City, CA). Thermal cycling was performed at 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles of amplification at 95°C for 15 seconds and then at 55°C for 1.5 minutes for denaturing and annealing, respectively.
Proliferation
In vitro proliferation was assayed using an in vitro fluorescence-based assay. After granuloma induction and immunization, magnetic activated cell sorting purified CD4+ T cells from lung lymph nodes were stained with 5 µmol/L 6-carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Inc., Eugene, OR) in PBS 5% fetal calf serum for 5 minutes at room temperature. Cells were washed several times to remove excess of CFSE and a total of 5 × 105 T cells were co-cultured with 1 × 105 cells bone marrow (BM)-derived macrophages and stimulated with SEA. BM was harvested from naive mice and filtered through nylon mesh. For generation of BM-derived macrophages, BM cells were cultured in L929 cell–conditioned medium as described previously.26 Six days after initial BM culture, BM-derived macrophages were transferred to 96-well plates for co-culture. After 96 hours of T-cell and macrophage co-culture at 37°C and 5% CO2, a minimum of 50,000 events on 5-(and 6-) carboxyfluorescein diacetate, succinimidyl ester T CD4+ cells were acquired on a dual-laser Cytomics FC500 flow cytometer (Beckman Coulter, Brea, CA) and analyzed using Flowjo software (TreeStar Inc., Ashland, OR). The software was used to calculate the percentage of dividing T cells.
Preparation of SEA
The S. mansoni eggs were grinded on ice for 40 minutes and the processed material was centrifuged at 60,000 rpm for 40 minutes to extract SEA. The supernatant was analyzed for protein content by Bradford assay, and the SEA subsequently was used for in vitro stimulation experiments.15
Statistical Analysis
Data are represented as mean ± SEM (n = 5) (PBS group) and were analyzed using GraphPad Prism version 5.0a for Mac (GraphPad Software, San Diego, CA). Comparisons were performed with one-way analysis of variance with Bonferroni's post-test. Differences were considered significant if the P value was <0.05. The experiments were repeated 2 times and we observed similar results in both experiments. All figures represent the results of one experiment.
Results
Histologic Alterations of Lung Parenchyma
To determine the effect of the DNA-hsp65 vaccine on the mobilization of inflammatory cells, we assessed granuloma size and composition during type 2 granuloma formation. At 8 days the granuloma was well defined in all groups but differences in size were observed. The granuloma was composed of mainly mononuclear cells and eosinophils after S. mansoni egg injection and DNA treatment did not modify this pattern. However, lungs of DNA-immunized mice were more preserved and the granulomas were smaller (Figure 1A). We observed that the DNA vaccine reduced collagen accumulation among perivascular and peribronchial areas 8 days after granuloma induction compared with vector or PBS control mice (Figure 1B), thus establishing potential antipathology effects of the vaccine. We further measured granuloma size (100 granulomas/group) and observed that the DNA treatment resulted in a 30% decrease in granuloma size compared with untreated or vector-treated groups (Figure 1C).
Figure 1.
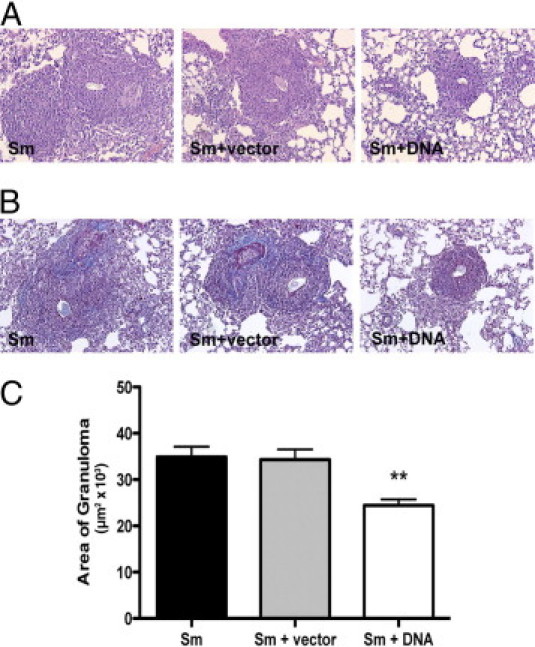
DNA-hsp65 immunization decreases type 2 granuloma size induced by injection of S. mansoni eggs. Mice were injected i.p. with 3000 eggs and after 14 days were immunized weekly with 3 doses of DNA-hsp65, vector control, or PBS. Seven days after the last DNA vaccine dose, mice received another 3000 eggs i.v. Eight days after egg injection, lung histology was evaluated. Histologic sections of lungs stained by H&E (A) and trichrome (B). C: Results of morphometry analysis. Data shown are mean ± SEM and are from a representative experiment of three independent experiments. **P < 0.01.
DNA-hsp65 Induces Reduction in the Number of Specific T Cells for Egg Antigens
To investigate how the DNA-hsp65 vaccine altered the cellular accumulation around the eggs in lung tissue, we accessed the proliferation of T cells from draining lymph nodes after in vitro stimulation with SEA. This antigen is described as a potent Th2 inducer during the granuloma formation.19 When CD4+ T cells from DNA-hsp65–immunized mice were stimulated with SEA, their in vitro proliferation was diminished compared with T cells from untreated or vector-treated mice, suggesting that DNA immunization induced a reduced number of SEA-specific Th2 cells (Figure 2), and thus may account for the smaller granuloma size in the lungs of these vaccinated mice.
Figure 2.
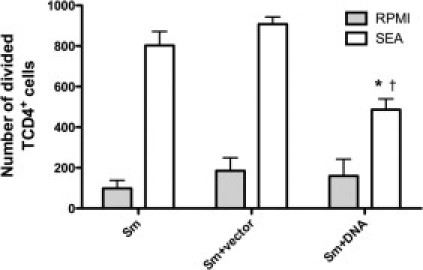
DNA-hsp65 immunization decreases the expansion of specific CD4+ T cells after type 2 granuloma induction. After granuloma induction and immunization, the CD4+ T cells from lung lymph nodes were purified using magnetic bead separation and were co-cultured with BM macrophages in the presence or absence of SEA. The T cells were incubated in the presence of 5-(and 6-) carboxyfluorescein diacetate, succinimidyl ester and the number of dividing cells was accessed by flow cytometry 96 hours after incubation. *P < 0.05 versus the nonimmunized group that received only eggs; †P < 0.05 versus the nonimmunized group that received only eggs + vector.
DNA-hsp65 Immunization Controls the Immune Response after Type 2 Granuloma Induction
After immunization and granuloma formation the transcript levels of Th1 and Th2 cytokines were evaluated. The DNA-hsp65 immunization induced an increase of genes related to Th1 expression and M1 macrophages because interferon (IFN)-γ, IL-12, and inducible nitric oxide synthase RNA levels were increased. On the other hand, arginase expression that is related to M2 activation, decreased with DNA treatment, thus showing a tendency to skew the Th2 pattern induced by eggs to a Th1 pattern (Figure 3).
Figure 3.
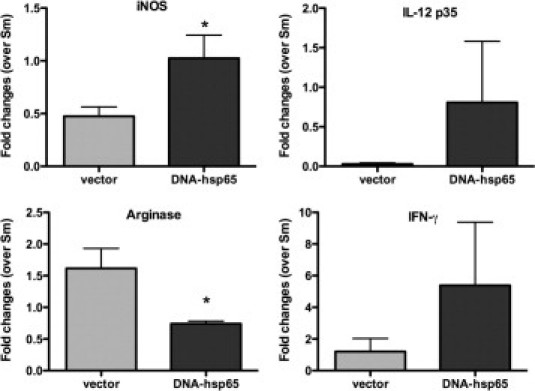
DNA-hsp65 immunization alters gene expression after type 2 granuloma induction. After granuloma induction and immunization, the RNA expression in lung tissue was analyzed. Data shown are mean ± SEM and are from a representative experiment of three independent experiments. *P < 0.05. iNOS, inducible nitric oxide.
Next, we evaluated the protein levels of cytokines associated with Th1 and Th2 responses. In lung homogenates of the nonimmunized group that received only eggs, high levels of IL-4, IL-13, and found in inflammatory zone-1 (FIZZ1), and lower levels of IL-12 and IFN-γ were found, indicating a skewing to a Th2 response (Figure 4). The DNA-hsp65 immunization changed this profile to a Th1 response as seen by the relative inhibition of IL-4, IL-13, and FIZZ-1, and the increases in IL-12 and IFN-γ production. Vector alone showed results similar to the S. mansoni egg group (nonimmunized group that received only eggs) except for IL-13 levels, which were lower, and INF-γ levels, which were higher. Those effects could be related to adjuvant properties of DNA vaccines.
Figure 4.
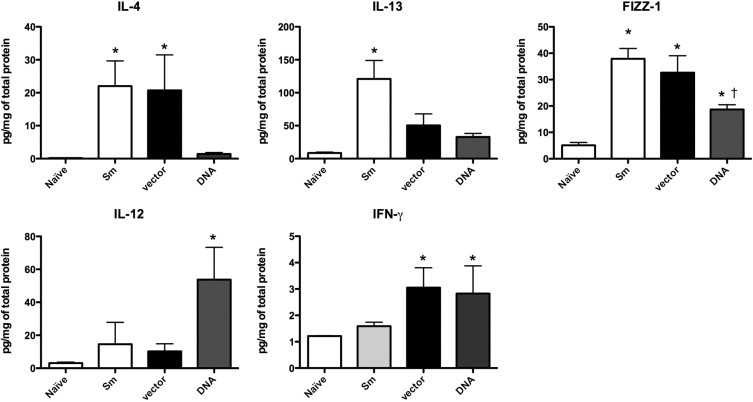
DNA-hsp65 immunization alters cytokines production after type 2 granuloma induction. After granuloma induction and immunization the presence of cytokines in lung homogenates was analyzed by a Bio-Plex bead-based cytokine assay (Bio-Rad). Data shown are mean ± SEM and are from a representative experiment of three independent experiments. *P < 0.05 versus naive group; †P < 0.05 versus nonimmunized group that received eggs only.
Effect of DNA Immunization on Fibrosis Induction
Because α-SMA+ myofibroblasts are recruited to the granuloma sites and deposit collagen leading to fibrosis we compared the amounts of this marker in lungs of DNA-vaccinated and control mice in our type 2 granuloma model. The DNA-hsp65 immunization induced a decreased migration of myofibroblasts to the lung granuloma periphery as visualized in lung sections stained with fluorescent antibodies (Figure 5A). In addition, mRNA levels of α-SMA and procollagen 1 were decreased in animals immunized with DNA-hsp65 (Figure 5B).
Figure 5.
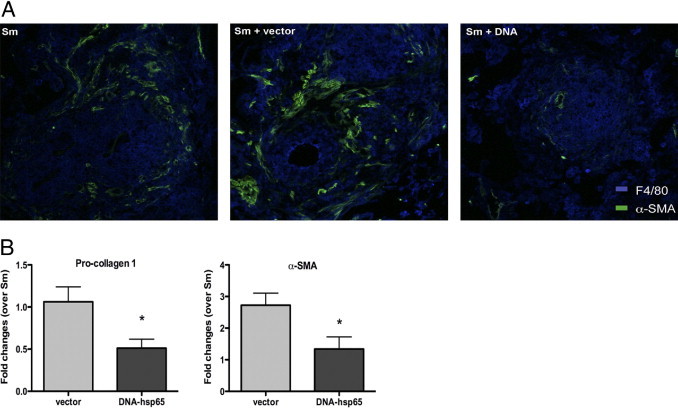
DNA-hsp65 immunization modulates fibrosis induction during type 2 granuloma formation. After immunization and granuloma induction lung sections were analyzed for α-SMA and procollagen 1 expression. A: Confocal immunofluorescent examination of pulmonary granulomas showed fewer α-SMA+ myofibroblasts (green) around F4/80+ cells (blue) in DNA-hsp65 immunized mice. Representative sections from one mouse of five per group are shown. Original magnification, ×200. B: Quantitative real-time PCR was performed to measure the transcript levels of procollagen 1 and α-SMA in whole lungs. Data shown are mean ± SEM and are from a representative experiment of three independent experiments. *P < 0.05.
Discussion
The hsp produced by Mycobacterium leprae (hsp65) has been the focus of our group as a DNA vaccine against several pathologies including TB,27,28 leishmaniasis,4 diabetes,6 arthritis,5 and cancer.7 For the first time, we are showing in this work the potential role of DNA-hsp65 as an immune modulator of pathology in a helminth infection, using an egg-induced granuloma model.
The cellular composition of the S. mansoni egg-induced granuloma includes eosinophils, macrophages, lymphocytes, neutrophils, mast cells, and fibroblasts.29 In human schistosomiasis there are typical granulomas, mainly in the liver, which invariably leads to tissue fibrosis represented by areas of chronic inflammation at the periphery of the portal vein system. This scenario culminates with the set of symptoms associated with the disease, including portal hypertension and blood congestion (owing to impaired blood flow by the eggs), hepatosplenomegaly, collateral circulation formation, and, finally, dissemination of eggs into the lungs, causing more granulomas.10 Therefore, our model of egg-induced granulomas is exclusively a lung model that could be extrapolated to human infection because the consequent pathologies are similar. Here, we have shown that previous immunization with DNA-hsp65 reduced the cellular recruitment around the eggs, and consequently decreased the granuloma area (Figure 1). Thus, our study describes a potential antipathology vaccine, regulating the damage signs induced by granulomatous diseases. This observation is relevant because the DNA vaccine did not block granuloma formation, which is crucial for containing the eggs and limiting pathogenesis, but decreased the inflammation, ensuring a preserved parenchyma necessary for major lung functions. Therefore, the control of pathology induced by exaggerated fibrosis is desirable during schistosomiasis and DNA-hsp65 seems to be a useful tool in this regard. We know that egg-induced pathology is regulated by type 2 cytokines,13 and, moreover, at the onset of granuloma-induced inflammation, the CD4+ T-cell response changes from a type 1 response of short duration to a sustained type 2 response,30 induced mainly by antigens secreted by Schistosoma eggs. Our results suggest that DNA immunization controlled the expansion of specific T cells to egg antigens, acting as a negative regulator of an exacerbated Th2 response. This result could explain the observed decrease in the size of granulomas because Th2 cells are often the main lymphocyte population in this structure.31 In agreement with the decrease of the Th2 skewed population after DNA treatment (Figure 2), we observed a decrease in IL-4 and IL-13 production in lungs of mice treated with DNA accompanied by increased production of IL-12 and IFN-γ (Figure 4), as well as increased expression of mRNA for IFN-γ and IL-12 (Figure 3). In previous work, using a TB model that classically induces a Th1 pattern, mice immunized with DNA-hsp65 showed increases of protective cytokines, IFN-γ and IL-12.3,32 Interestingly, in this work we showed that besides the strong Th2 polarization induced by S. mansoni eggs, DNA-hsp65 was able to maintain increased levels of IFN-γ and IL-12 while simultaneously decreasing the release of Th2-related cytokines (Figure 4). This polarization of the immune response induced by DNA treatment could be associated with the size of lung lesions observed (Figure 1), as well as with differential activation of macrophages in the site of granulomas (Figure 3). It is well known that IFN-γ primes macrophages via the Janus kinase1/2-STAT1 pathway, causing the classic type 1 activation (M1 macrophages) that induces a set of transcription factors ultimately leading to up-regulated inducible nitric oxide synthase expression and down-regulated arginase expression.33 Here, we show a significant expression of M1-related phenotype with high inducible nitric oxide synthase and low arginase levels (Figure 3) after DNA treatment associated with high levels of IFN-γ and reduced levels of FIZZ-1 (Figure 4), suggesting that DNA-hsp65 exerts a crucial role in directing the cell differentiation in this model.
Some of the pathologic changes seen during type 2 granuloma formation are owing to the presence of Th2 cytokine–driven responses that recruit and activate eosinophils, dendritic cells, CD4+ Th2 cells, and, alternatively, activated macrophages,34 and that synthesize or induce the synthesis of profibrotic factors in the course of an inflammatory process.35 This granuloma model showed the development of a fibrotic milieu in lung tissue, with collagen accumulation inside the granuloma (Figure 1), high expression of procollagen 1 and α-SMA (Figure 5), and high production of IL-13 (Figure 4), a key cytokine that mediates fibrosis in pulmonary disease.36 Nevertheless, DNA therapy controlled this scenario, reducing the IL-13 production (Figure 4) and procollagen 1 and α-SMA mRNA expression (Figure 5B), as well as a decrease of α-SMA protein in lung tissue (Figure 5A).
Finally, previous work has shown the antifibrotic effects of DNA-hsp65 vaccine in a pulmonary fibrosis model induced by bleomycin, in which the vaccine reduced the deposition of the noncollagenous matrix in the lungs of mice, composed mainly of elastin, proteoglycans, laminin, and fibronectin.37 Here, we present a new effect of this DNA vaccine, one of reducing the collagen deposition in a type 2 granuloma model. Taken together, our results indicate that the adjuvant properties of DNA-hsp65 vaccine regulate the immune response in this Th2 model, leading to preserved lung parenchyma. These findings suggest that the DNA-hsp65 vaccine can be used as a tool to control the fibrosis process in diverse pathologic diseases, including schistosomiasis. Further, its clinical relevance needs to be tested in combination with standard therapies to see if additive or even synergistic affects are possible.
Acknowledgment
We thank Dr. Judith Connett for her careful reading of this manuscript.
Footnotes
Supported by The Foundation for Research Support of São Paulo (FAPESP) (2003/12887-8); and by two grants from the National Institutes of Health, National Heart, Lung, and Blood Institute: #HL089216/HL/NHLBI NIH HHS/United States, and HL031963/HL/NHLBI NIH HHS/United States.
References
- 1.de Paula L., Silva C.L., Carlos D., Matias-Peres C., Sorgi C.A., Soares E.G., Souza P.R., Blades C.R., Galleti F.C., Bonato V.L., Goncalves E.D., Silva E.V., Faccioli L.H. Comparison of different delivery systems of DNA vaccination for the induction of protection against tuberculosis in mice and guinea pigs. Genet Vaccines Ther. 2007;5:2. doi: 10.1186/1479-0556-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosada R.S., Torre L.G., Frantz F.G., Trombone A.P., Zarate-Blades C.R., Fonseca D.M., Souza P.R., Brandao I.T., Masson A.P., Soares E.G., Ramos S.G., Faccioli L.H., Silva C.L., Santana M.H., Coelho-Castelo A.A. Protection against tuberculosis by a single intranasal administration of DNA-hsp65 vaccine complexed with cationic liposomes. BMC Immunol. 2008;9:38. doi: 10.1186/1471-2172-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva C.L., Bonato V.L., Coelho-Castelo A.A., De Souza A.O., Santos S.A., Lima K.M., Faccioli L.H., Rodrigues J.M. Immunotherapy with plasmid DNA encoding mycobacterial hsp65 in association with chemotherapy is a more rapid and efficient form of treatment for tuberculosis in mice. Gene Ther. 2005;12:281–287. doi: 10.1038/sj.gt.3302418. [DOI] [PubMed] [Google Scholar]
- 4.Coelho E.A., Tavares C.A., Lima Kde M., Silva C.L., Rodrigues J.M., Jr, Fernandes A.P. Mycobacterium hsp65 DNA entrapped into TDM-loaded PLGA microspheres induces protection in mice against Leishmania (Leishmania) major infection. Parasitol Res. 2006;98:568–575. doi: 10.1007/s00436-005-0088-5. [DOI] [PubMed] [Google Scholar]
- 5.Santos-Junior R.R., Sartori A., De Franco M., Filho O.G., Coelho-Castelo A.A., Bonato V.L., Cabrera W.H., Ibanez O.M., Silva C.L. Immunomodulation and protection induced by DNA-hsp65 vaccination in an animal model of arthritis. Hum Gene Ther. 2005;16:1338–1345. doi: 10.1089/hum.2005.16.1338. [DOI] [PubMed] [Google Scholar]
- 6.Santos Junior R.R., Sartori A., Bonato V.L., Coelho Castelo A.A., Vilella C.A., Zollner R.L., Silva C.L. Immune modulation induced by tuberculosis DNA vaccine protects non-obese diabetic mice from diabetes progression. Clin Exp Immunol. 2007;149:570–578. doi: 10.1111/j.1365-2249.2007.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaluart P., Abdallah K.A., Lima F.D., Smith R., Moyses R.A., Coelho V., Victora G.D., Socorro-Silva A., Volsi E.C., Zarate-Blades C.R., Ferraz A.R., Barreto A.K., Chammas M.C., Gomes R., Gebrim E., Arakawa-Sugueno L., Fernandes K.P., Lotufo P.A., Cardoso M.R., Kalil J., Silva C.L. Phase I trial of DNA-hsp65 immunotherapy for advanced squamous cell carcinoma of the head and neck. Cancer Gene Ther. 2008;15:676–684. doi: 10.1038/cgt.2008.35. [DOI] [PubMed] [Google Scholar]
- 8.Frantz F.G., Rosada R.S., Peres-Buzalaf C., Perusso F.R., Rodrigues V., Ramos S.G., Kunkel S.L., Silva C.L., Faccioli L.H. Helminth coinfection does not affect therapeutic effect of a DNA vaccine in mice harboring tuberculosis. PLoS Negl Trop Dis. 2010;4:e700. doi: 10.1371/journal.pntd.0000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boros D.L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- 10.Boros D.L. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev. 1989;2:250–269. doi: 10.1128/cmr.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheever A.W. Schistosomiasis infection versus disease and hypersensitivity versus immunity. Am J Pathol. 1993;142:699–702. [PMC free article] [PubMed] [Google Scholar]
- 12.Metwali A., Elliott D., Blum A.M., Li J., Sandor M., Lynch R., Noben-Trauth N., Weinstock J.V. The granulomatous response in murine Schistosomiasis mansoni does not switch to Th1 in IL-4-deficient C57BL/6 mice. J Immunol. 1996;157:4546–4553. [PubMed] [Google Scholar]
- 13.Chensue S.W., Warmington K., Ruth J., Lincoln P., Kuo M.C., Kunkel S.L. Cytokine responses during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation: Production of Th1 and Th2 cytokines and relative contribution of tumor necrosis factor. Am J Pathol. 1994;145:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu B.C., Freeman C.M., Stolberg V.R., Komuniecki E., Lincoln P.M., Kunkel S.L., Chensue S.W. Cytokine-chemokine networks in experimental mycobacterial and schistosomal pulmonary granuloma formation. Am J Respir Cell Mol Biol. 2003;29:106–116. doi: 10.1165/rcmb.2002-0241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi A.D., Schaller M.A., Lukacs N.W., Kunkel S.L., Hogaboam C.M. TLR3 modulates immunopathology during a Schistosoma mansoni egg-driven Th2 response in the lung. Eur J Immunol. 2008;38:3436–3449. doi: 10.1002/eji.200838629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez H.J., Trzyna W.C., Cordingley J.S., Brodeur P.H., Stadecker M.J. Differential antigen recognition by T cell populations from strains of mice developing polar forms of granulomatous inflammation in response to eggs of Schistosoma mansoni. Eur J Immunol. 1997;27:666–670. doi: 10.1002/eji.1830270314. [DOI] [PubMed] [Google Scholar]
- 17.Pearce E.J., Caspar P., Grzych J.M., Lewis F.A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth: Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukacs N.W., Boros D.L. Utilization of fractionated soluble egg antigens reveals selectively modulated granulomatous and lymphokine responses during murine schistosomiasis mansoni. Infect Immun. 1992;60:3209–3216. doi: 10.1128/iai.60.8.3209-3216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearce E.J., Kane M.C., Sun J., Taylor J.J., McKee A.S., Cervi L. Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunol Rev. 2004;201:117–126. doi: 10.1111/j.0105-2896.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 20.Scotton C.J., Chambers R.C. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 21.Lavergne F., Clerici C., Sadoun D., Brauner M., Battesti J.P., Valeyre D. Airway obstruction in bronchial sarcoidosis: outcome with treatment. Chest. 1999;116:1194–1199. doi: 10.1378/chest.116.5.1194. [DOI] [PubMed] [Google Scholar]
- 22.Bonato V.L., Goncalves E.D., Soares E.G., Santos R.R., Jr, Sartori A., Coelho-Castelo A.A., Silva C.L. Immune regulatory effect of pHSP65 DNA therapy in pulmonary tuberculosis: activation of CD8+ cells, interferon-gamma recovery and reduction of lung injury. Immunology. 2004;113:130–138. doi: 10.1111/j.1365-2567.2004.01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuomela M., Stanescu I., Krohn K. Validation overview of bio-analytical methods. Gene Ther. 2005;12(Suppl 1):S131–S138. doi: 10.1038/sj.gt.3302627. [DOI] [PubMed] [Google Scholar]
- 24.Ito T., Schaller M., Hogaboam C.M., Standiford T.J., Chensue S.W., Kunkel S.L. TLR9 activation is a key event for the maintenance of a mycobacterial antigen-elicited pulmonary granulomatous response. Eur J Immunol. 2007;37:2847–2855. doi: 10.1002/eji.200737603. [DOI] [PubMed] [Google Scholar]
- 25.Wen H., Hogaboam C.M., Gauldie J., Kunkel S.L. Severe sepsis exacerbates cell-mediated immunity in the lung due to an altered dendritic cell cytokine profile. Am J Pathol. 2006;168:1940–1950. doi: 10.2353/ajpath.2006.051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi A.D., Raymond T., Coelho A.L., Kunkel S.L., Hogaboam C.M. A systemic granulomatous response to Schistosoma mansoni eggs alters responsiveness of bone-marrow-derived macrophages to Toll-like receptor agonists. J Leukoc Biol. 2008;83:314–324. doi: 10.1189/jlb.1007689. [DOI] [PubMed] [Google Scholar]
- 27.de Paula L., Silva C.L., Carlos D., Matias-Peres C., Sorgi C.A., Soares E.G., Souza P.R., Blades C.R., Galleti F.C., Bonato V.L., Goncalves E.D., Silva E.V., Faccioli L.H. Comparison of different delivery systems of DNA vaccination for the induction of protection against tuberculosis in mice and guinea pigs. Genet Vaccines Ther. 2007;5:2. doi: 10.1186/1479-0556-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosada R.S., de la Torre L.G., Frantz F.G., Trombone A.P., Zarate-Blades C.R., Fonseca D.M., Souza P.R., Brandao I.T., Masson A.P., Soares E.G., Ramos S.G., Faccioli L.H., Silva C.L., Santana M.H., Coelho-Castelo A.A. Protection against tuberculosis by a single intranasal administration of DNA-hsp65 vaccine complexed with cationic liposomes. BMC Immunol. 2008;9:38. doi: 10.1186/1471-2172-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan M.H., Whitfield J.R., Boros D.L., Grusby M.J. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J Immunol. 1998;160:1850–1856. [PubMed] [Google Scholar]
- 30.Raymond T., Schaller M., Hogaboam C.M., Lukacs N.W., Rochford R., Kunkel S.L. Toll-like receptors: Notch ligands, and cytokines drive the chronicity of lung inflammation. Proc Am Thorac Soc. 2007;4:635–641. doi: 10.1513/pats.200706-067TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearce E.J., MacDonald A.S. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 32.Goncalves E.D., Bonato V.L., Fonseca D.M., Soares E.G., Brandao I.T., Soares A.P., Silva C.L. Improved protective efficacy of a TB DNA-HSP65 vaccine by BCG priming. Genet Vaccines Ther. 2007;5:7. doi: 10.1186/1479-0556-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma J., Chen T., Mandelin J., Ceponis A., Miller N.E., Hukkanen M., Ma G.F., Konttinen Y.T. Regulation of macrophage activation. Cell Mol Life Sci. 2003;60:2334–2346. doi: 10.1007/s00018-003-3020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito T., Schaller M., Raymond T., Joshi A.D., Coelho A.L., Frantz F.G., Carson W.F., IV, Hogaboam C.M., Lukacs N.W., Standiford T.J., Phan S.H., Chensue S.W., Kunkel S.L. Toll-like receptor 9 activation is a key mechanism for the maintenance of chronic lung inflammation. Am J Respir Crit Care Med. 2009;180:1227–1238. doi: 10.1164/rccm.200906-0892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 36.Saw V.P., Offiah I., Dart R.J., Galatowicz G., Dart J.K., Daniels J.T., Calder V.L. Conjunctival interleukin-13 expression in mucous membrane pemphigoid and functional effects of interleukin-13 on conjunctival fibroblasts in vitro. Am J Pathol. 2009;175:2406–2415. doi: 10.2353/ajpath.2009.090579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padua A.I., Silva C.L., Ramos S.G., Faccioli L.H., Martinez J.A. Influence of a DNA-hsp65 vaccine on bleomycin-induced lung injury. J Bras Pneumol. 2008;34:891–899. doi: 10.1590/s1806-37132008001100002. [DOI] [PubMed] [Google Scholar]


