Figure 1.
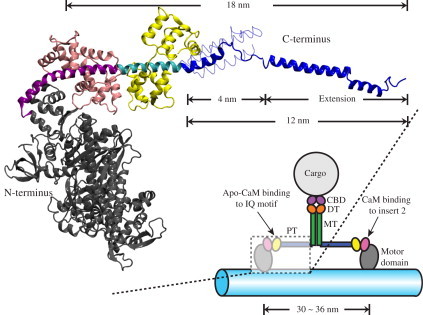
Structure of myosin VI. (Lower right) Schematic of the myosin VI dimer moving on an F-actin filament (light blue). Myosin VI consists of an N-terminal motor domain (gray) followed by insert-2 and IQ motifs, which bind calmodulin (CaM; pink) and apo-CaM (yellow), respectively, and are occluded in the schematic. The IQ motif is followed by the proximal tail (PT) domain (blue), medial tail (MT) domain (green), and distal tail (DT) domain (orange). The C-terminal cargo binding domain (CBD; purple) associates with the cargo. (Upper left) Expanded view of a portion of myosin VI in cartoon representation with the same color scheme as in the lower-right schematic. Insert-2 (purple) and IQ motif (cyan) are shown explicitly. The model shown was built by fusing two x-ray structures (PDB codes: 2BKI (21) and 3GN4 (22)). The PT domain (blue) is shown in its extended form, i.e., with its three α-helices extended to a full length of 12 nm, being capable then together with the dimer partner of a 30–36-nm step size (see text) (22). The PT domain is also shown in its unextended, 4-nm-long, three-helix zig-zag bundle form (22) in thin tube representation (see also Fig. 3D). The extended conformation of the PT domain shown is a key result from this study (see Results).
