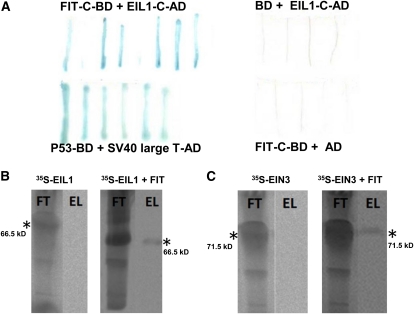
Figure 1.
Yeast and in Vitro Protein Interaction of FIT with EIL1 and EIN3.
(A) In yeast; lacZ assays after retransformation. FIT-C fused to Gal4 DNA binding domain (FIT-C-BD) with EIL1-C peptide fused to activation domain (EIL1-C-AD) (top left, positive interaction resulting in blue staining); unfused BD with EIL1-C-AD (top right, negative control, white staining); P53-BD with SV40 large T antigen-AD (bottom left, positive control, blue staining); and FIT-C-BD with unfused AD (bottom right, negative control, white staining).
(B) In vitro protein pull down of 35S-labeled EIL1 by S-tagged FIT, detected by immunoblot and phosphor imaging (right side), and in the absence of S-tagged FIT (left side, negative control).
(C) As in (B) with 35S-labeled EIN3. FT, flow-through fraction after binding and loading onto S-agarose column; EL, eluate fraction containing radioactive protein interaction partner. Asterisk indicates the respective positions and sizes of the radiolabeled proteins.
[See online article for color version of this figure.]