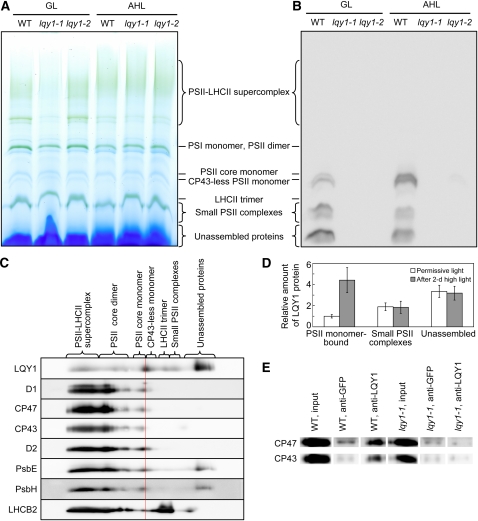
Figure 6.
Evidence for Interaction of LQY1 Protein with PSII Protein Complexes.
(A) A representative unstained BN-PAGE gel. Thylakoid membranes were solubilized with 1.5% digitonin and separated on a NativePAGE 3 to 12% Bis-Tris mini gel. A sample with an equal amount of chlorophyll (7 μg) was loaded in each lane. GL, growth light; AHL, after 2 d of high light; WT, wild type.
(B) A representative immunoblot with anti-LQY1 antiserum of a gel as in (A). The positions of various PSII protein complexes were determined by immunoblotting with antisera against PSII core proteins D1 and CP43. Little signal is observed in lqy1 mutants, validating the specificity of the antibody.
(C) 2-D electrophoresis and immunoblots of PSII protein complexes from Col wild-type leaves after a 2-d high-light treatment. Protein complexes were separated as in (A) by BN-PAGE (first dimension), and proteins in the complexes were separated by SDS-PAGE (second dimension). The 2-D gel was electroblotted and probed with antisera against LQY1 and various PSII proteins. The positions of detected PSII protein complexes are indicated. The red line separates the CP43-less monomer from the PSII core monomer.
(D) Relative abundance of LQY1 protein in Col wild-type plants as detected in (B). The values (mean ± se, n = 3) are given as the ratio to PSII monomer-bound LQY1 in Col wild-type plants under growth light. Note that in Figure 6B, the relative intensity of LQY1 in small PSII complexes from wild-type plants decreased after a 2-d high-light treatment. However, this is not seen in Figure 6D, which represents the mean values from three independent BN-PAGE/immunoblots.
(E) Immunoprecipitation of CP47 and CP43 by anti-LQY1 antibody. Thylakoid membranes were extracted from Col wild-type and lqy1-1 leaves after a 2-d high-light treatment.