Figure 6.
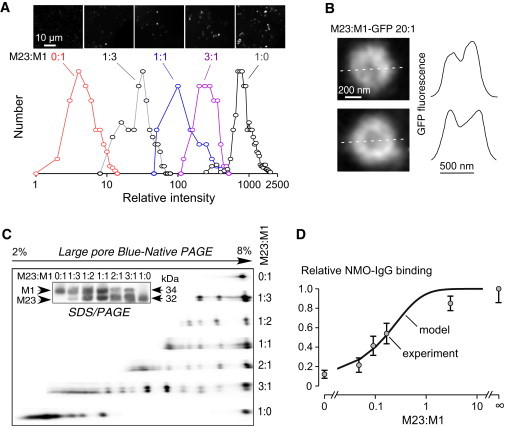
Experimental measurements of OAP properties and NMO-IgG/AQP4 binding. (A) U87MG cells were transfected with GFP-M23 and GFP-M1 AQP4 at indicated ratios. Representative TIRF micrographs show fluorescent spot (top). Deduced number histograms of single spot fluorescence (background-subtracted, area-integrated intensities), proportional to AQP4 aggregate (OAP) size, shown at the bottom. Unity represents the intensity of monomeric GFP. (B) U87MG cells were transfected with (untagged) M23 AQP4 and GFP-M1 AQP4 and at a ratio of 20:1. Representative TIFR micrographs of large AQP4 aggregate (left) show relative concentration of fluorescence at the periphery. Line profiles (dashed white lines at the left) shown at the right. (C) AQP4 immunoblot after SDS/PAGE (inset) and BN-SDS/PAGE of lysates from U87MG cells expressing (untagged) M1 and M23 AQP4 at indicated ratios. (D) NMO-IgG binding to AQP4 OAPs. U87MG cells expressing M1 and M23 AQP4 (both untagged) were stained with NMO-IgG (recombinant monoclonal antibody rAb-53(28)) and anti-AQP4 antibody as described under Experimental Procedures section. Relative binding (normalized to unity at M23:M1 = ∞) measured by quantitative ratio imaging (SE n = 4) shown along with model-predicted curve: relative binding = 0.14 + 0.86 [1-(1+α)-4], where α is the M23:M1 ratio; [1-(1+α)-4] is the probability that an AQP4 monomer is OAP-associated.
