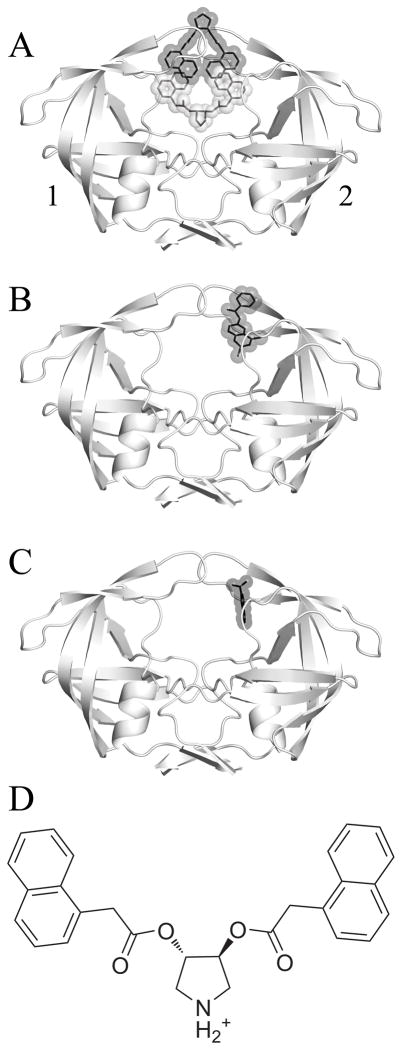
Figure 1.
A) The crystallized HIV-1 protease uniquely bound by two identical inhibitors, with pose α colored in grey and pose β in black. B) The crystal structure 3BC4 with Damm compound 112 (black) bound at the eye site. C) The 5-Nitroindole fragment (black) crystallized in the eye site by Perryman et al.28 D) A 2-dimensional representation of the pyrrolidine inhibitor that was co-crystallized with 3BC4. The affinity (Ki) of the compound for HIVp was measured by Klebe and coworkers as 20μM (WT), 41μM (I50V), and 4.5μM (I84V).11 For the following figures, we have used a convention of orienting the complex so that a naphthyl occupies the eye position on the right (ie. Monomer 2). We are labeling the monomers as “1” and “2” instead of “A” and “B” to avoid confusion with the α and β notation for the ligands.