Figure 1.
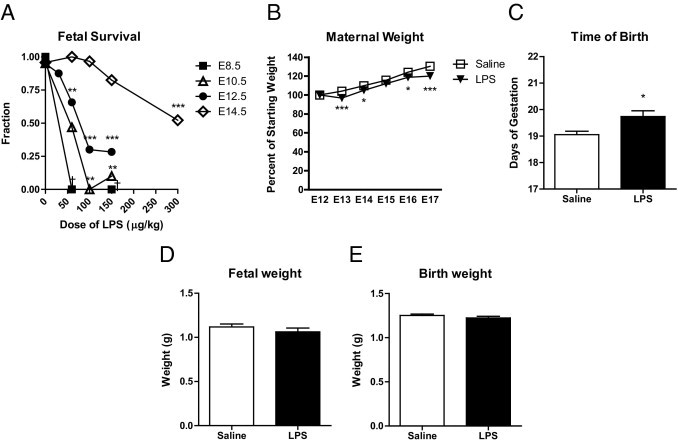
Dose-dependent risk of fetal loss but lack of growth restriction or premature birth after maternal LPS administration in a mouse model. A: The number of surviving fetuses was counted 24 hours after treatment with LPS at different embryonic ages and doses (n = 3 to 40 pregnancies per group). The effect of treatment on survival was significant at E10.5 (P = 0.0022), E12.5 (P < 0.0001), and E14.5 (P < 0.0001) by one-way analysis of variance. Analysis of variance could not be performed for E8.5 treatments because of lack of variance within groups. **P < 0.01; ***P < 0.001 by Dunnett's multiple comparison post hoc test. B: Pregnant mice were treated with saline (n = 13) or LPS (60 μg/kg) (n = 17) on E12.5. Maternal weights were monitored daily and were expressed as a percentage of starting weight at the time of treatment. LPS-treated maternal weights are slightly but significantly lower than for saline-injected control animals (P < 0.0001 by two-way analysis of variance). *P < 0.05; ***P < 0.001 by Bonferroni post hoc test). C: Pregnant mice were treated with saline (n = 18) or LPS (60 μg/kg) (n = 21) on E12.5 and the time of birth was recorded. The length of gestation in LPS-injected animals was increased by approximately 0.5 days. *P = 0.014 by two-tailed t-test. D and E: Pregnant mice were treated with saline (n = 36) or LPS (60 μg/kg) (n = 31) on E12.5. Fetuses were collected and weighed at E17.5 or at birth. Fetal growth was unaffected in LPS-treated pregnancies (E17.5: P = 0.30 by two-tailed t-test; birth: P = 0.27 by two-tailed t-test). Data are reported as means ± SEM.
