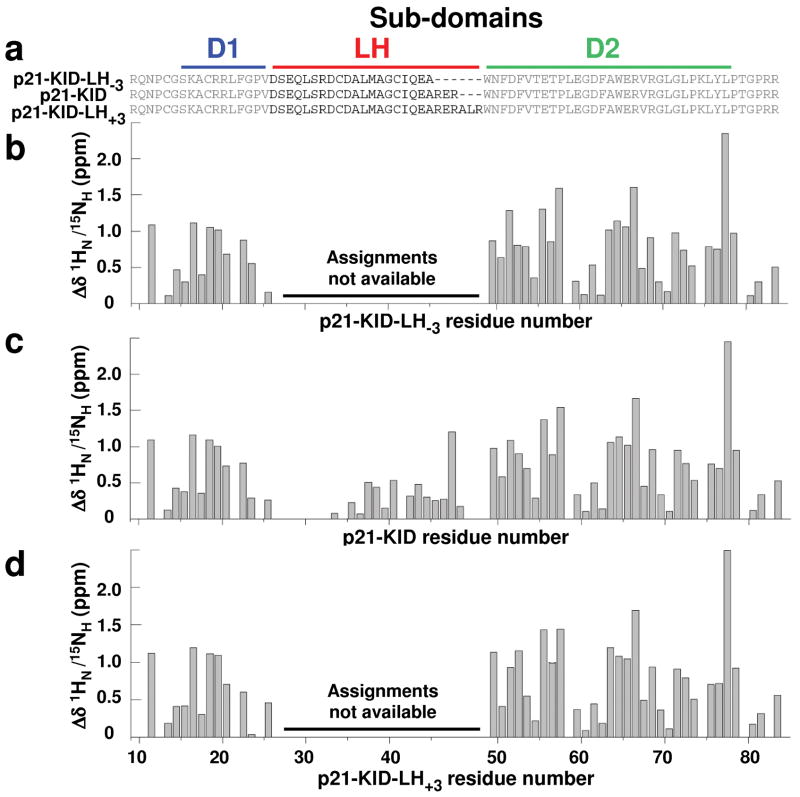
Figure 3.
The Cdk2/cyclin A-bound structures of sub-domains D1 and D2 within the p21 constructs are unaffected by elongation or truncation of sub-domain LH by 3 residues. (a) Sequences of wild-type p21-KID and the variants with a lengthened (p21-KID-LH+3) and shortened (p21-KID-LH−3) LH sub-domain. Differences between experimental composite 1HN/15NH chemical shift values for residues in (b) p21-KID-LH−3, (c) p21-KID, and (d) p21-KID-LH+3 when bound to Cdk2/cyclin A and sequence-adjusted, standard values for residues in random coil peptides (Δδ 1HN/15NH). The magnitude of these Δδ 1HN/15NH values reflects the extent of structural change that occurs when the p21 constructs fold upon binding to Cdk2/cyclin A.