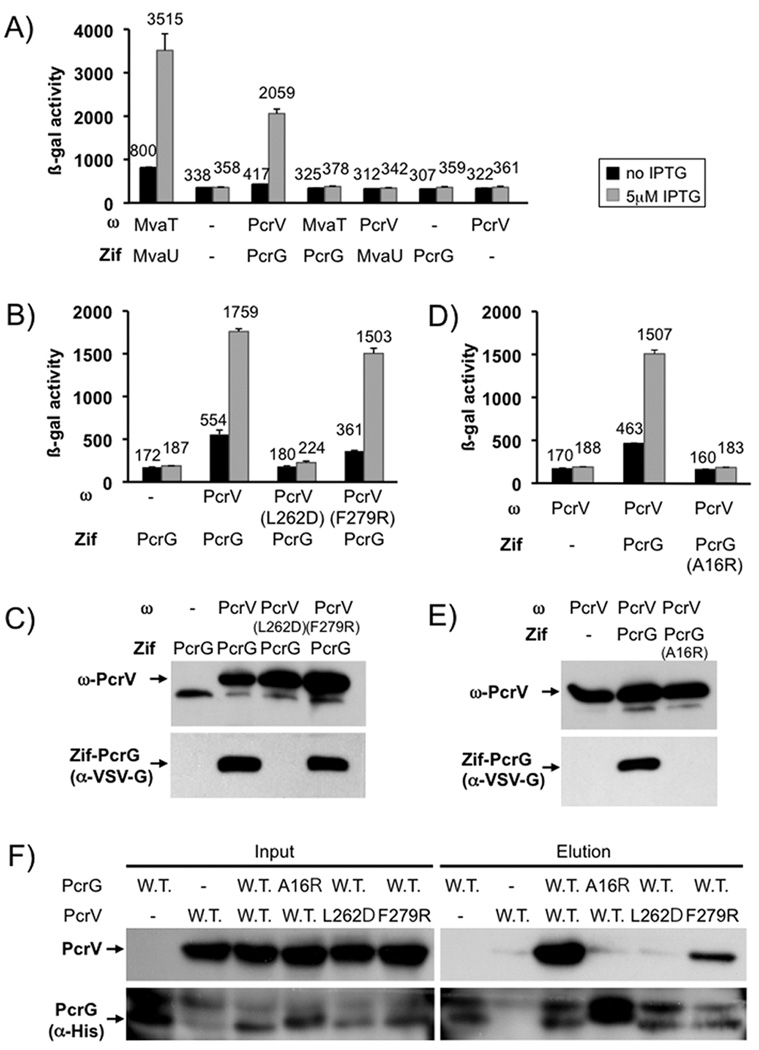
Fig. 5. The interaction between PcrG and PcrV.
The interaction between PcrG and PcrV was assayed by two-hybrid analysis. PcrV was fused to the omega-subunit of RNA polymerase (ω). PcrG was fused to the monomeric DNA binding protein Zif. Interaction of the fusion proteins results in recruitment of RNA polymerase to a test promoter, which can be readily assayed by β-galactosidase assay. PcrG and PcrV interact with each other and not the unrelated histone-like proteins MvaT and MvaU (A). The effect of mutations in PcrV (B) or PcrG (D) on the interaction was monitored by using the same two-hybrid system, the only modification being that the Zif-fusion had been modified by inserting a VSV-G tag into the linker between Zif and PcrG. Production of the ω and Zif fusion proteins was monitored by western blot (C, E). Interaction data was confirmed by pull-down in E. coli (F). PcrV and a His-tagged version of PcrG, as well as mutants of either protein, were co-expressed in E. coli BL21. Co-purification of PcrG and PcrV was monitored by detecting PcrV in the elution fraction after purification.