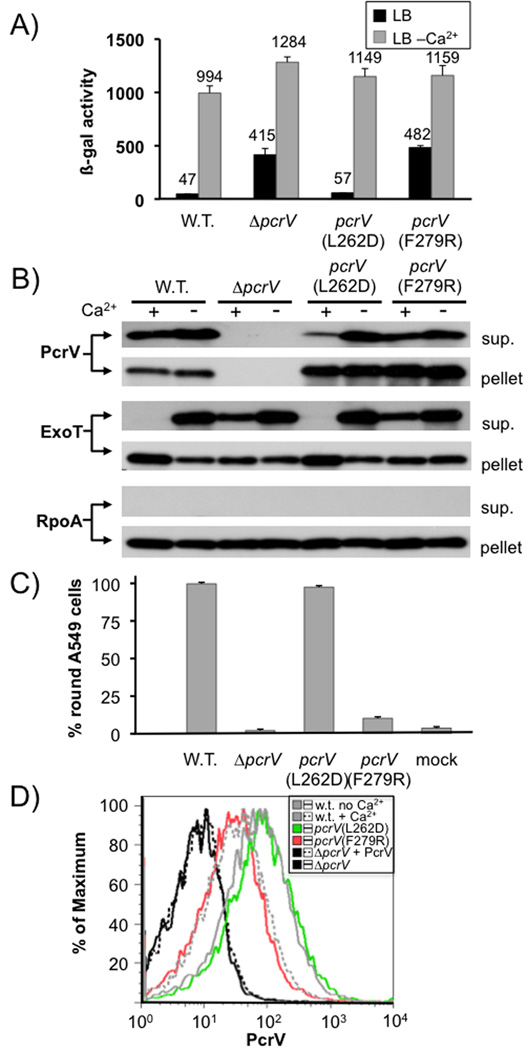
Fig. 6. Phenotypic characterization of pcrV point mutants.
The effect of point mutations L262D and F279R in pcrV was assayed by β-galactosidase assay using a chromosomal lacZ reporter inserted in the exoS locus (A). It was also examined by RECC assay (B). Secretion of PcrV, ExoT and RpoA (control) was monitored by Western blot (the respective protein detected is indicated to the left of each blot, the fraction (cell pellet or supernatant) is indicated to the right). The ability to intoxicate cells was determined by monitoring rounding of A549 cells after 5 hours of infection (MOI10). Surface localization of PcrV was monitored by FACS using an affinity-purified anti-PcrV antiserum and an anti-rabbit IgG APC-conjugated secondary antibody. To normalize expression of PcrV, all strains were grown in the absence of calcium, except the wild-type, which was grown both in the absence and presence of calcium (indicated in the legend). Purified PcrV protein was added to one sample of the pcrV null mutant before performing the antibody staining procedure in order to rule out the possibility that secreted protein being non-specifically cross-linked to the cells interfered with our assay.