Abstract
Background
Counseling patients with enhancing renal mass currently occurs in the context of significant uncertainty regarding tumor pathology.
Objective
We evaluated whether radiographic features of renal masses could predict tumor pathology and developed a comprehensive nomogram to quantitate the likelihood of malignancy and high-grade pathology based on these features.
Design, setting, and participants
We retrospectively queried Fox Chase Cancer Center’s prospectively maintained database for consecutive renal masses where a Nephrometry score was available.
Intervention
All patients in the cohort underwent either partial or radical nephrectomy.
Measurements
The individual components of Nephrometry were compared with histology and grade of resected tumors. We used multiple logistic regression to develop nomograms predicting the malignancy of tumors and likelihood of high-grade disease among malignant tumors.
Results and limitations
Nephrometry score was available for 525 of 1750 renal masses. Nephrometry score correlated with both tumor grade (p < 0.0001) and histology (p < 0.0001), such that small endophytic nonhilar tumors were more likely to represent benign pathology. Conversely, large interpolar and hilar tumors more often represented high-grade cancers. The resulting nomogram from these data offers a useful tool for the preoperative prediction of tumor histology (area under the curve [AUC]: 0.76) and grade (AUC: 0.73). The model was subjected to out-of-sample cross-validation; however, lack of external validation is a limitation of the study.
Conclusions
The current study is the first to objectify the relationship between tumor anatomy and pathology. Using the Nephrometry score, we developed a tool to quantitate the preoperative likelihood of malignant and high-grade pathology of an enhancing renal mass.
Keywords: Kidney, Carcinoma, Renal cell, Nomograms, Nephrometry, Anatomy
1. Introduction
Pathologic uncertainty exists when an incidental renal mass is identified. Preoperative counseling and treatment planning are often made in the context of this uncertainty, despite the fact that 20–30% of these lesions ultimately prove benign and only 10–30% are found to be potentially aggressive [1-4]. As such, the rising rates of renal surgery for the small renal mass (SRM) have yet to translate into a quantifiable survival benefit [5]. Although efforts have been made to assess malignant potential using preoperative variables, to date the clinical usefulness of nonextirpative diagnostic strategies including percutaneous biopsy and pathologic predictive models remains limited [6-8].
Increasing evidence suggests a relationship may exist between renal mass anatomy and pathology [9-11]; however, only recently have objective measures of defining renal mass anatomy been described [12-14]. Nephrometry is the first such scoring system to allow quantification of renal tumor anatomy in a reproducible manner [12]. Here we determined whether anatomic radiographic attributes of renal masses, as scored by Nephrometry, can predict the pathologic characteristics of enhancing renal masses. We also developed a predictive model integrating renal tumor anatomy with other demographic characteristics to predict tumor histology and grade.
2. Methods
Using the prospectively maintained Kidney Cancer Keystone database approved by our institutional review board, we identified all patients who underwent renal surgery at our institution with available Nephrometry scores. Demographic, clinical, pathologic, and cross-sectional imaging characteristics were reviewed for all identified patients. Renal cell carcinoma stage was assigned by surgical pathology according to the American Joint Committee on Cancer 2002 TNM classification [15]. For the purposes of statistical analysis, tumors with Fuhrman grade I and II were considered low grade, and grade III and IV tumors were classified as high grade. Tumor histology was assigned according to the 2004 World Health Organization criteria [16].
Nephrometry scores (www.nephrometry.com; Table 1) [12] have been calculated and input prospectively since 2009. To assess the relationship of histology with the Nephrometry components, we used multiple linear regression for total Nephrometry score, multinomial logistic regression (for R, E, N, A, L components) and logistic regression (for H score). The p values came from the F test (linear regression) or likelihood ratio test (logistic regressions) comparing the model with the histology components entered via dummy variables with the intercept-only model.
Table 1.
– Description of the RENAL Nephrometry Score*
| 1 point | 2 points | 3 points | |
|---|---|---|---|
| Radius, maximal diameter, cm | ≤4 | >4 but <7 | ≥7 |
| Exophytic properties | ≥ 50% | <50% | Entirely endophytic |
| Nearness of the tumor to the collecting system or sinus, mm | ≥7 | >4 but <7 | ≤4 |
| Anterior/posterior | No points given. Mass assigned a descriptor of A, P, or X | ||
| Location relative to the polar lines† | Entirely above the upper or below the lower polar line | Lesion crosses the polar line | >50% of the mass is across polar line or the mass crosses the axial renal midline or the mass is entirely between the polar lines |
Adopted from Kutikov and Uzzo [12]. Web tool for point-of-service use can be found at www.nephrometry.com.
Suffix “h” assigned if the tumor touches the main renal artery or vein.
Multiple logistic regression analyses were used to identify clinical and anatomic variables associated with malignant histology and high-grade features among those with malignant histology at the time of surgical resection. Covariates included in the multiple logistic models of malignant histology and grade included patient sex, age, and individual anatomic components of the Nephrometry scoring system. Marginally statistically significant sex and age associations at the p < 0.10 level were retained in the nomogram models to assure that we were appropriately controlling for possible confounding variables. Age was entered into the model via the use of restricted cubic splines with three knots placed at empirical quantiles [17].
The predictive accuracy of the nomograms was quantified by receiver operating characteristic derived area under the curve (AUC) estimates with 100% indicating perfect prediction and 50% equivalent to a coin toss. Nested nomogram models were compared using likelihood ratio tests. To evaluate the predictive characteristics of our nomograms more generally, we performed a simulation with 10 000 iterations in which we randomly split the sample into a training and test data set with each iteration (50% split with each iteration). The nomogram models were fit in the training sample, and the AUC was calculated on the test data set using the training data-set model.
To assess the calibration of our models, we used a “leave one out prediction” technique in which for each person we predicted their outcomes after using the rest of the sample’s data to fit our models. We then grouped individuals into quintiles based on their predicted probabilities of having cancer or high-grade disease. Next, we examined the proportion within each quintile that actually had cancer or high-grade disease. We used a scatter plot of the observed versus average predicted proportions within each quintile in which the points should lie on a 45% line. Similar calibration was described previously [18].
The analyses were conducted using Stata v.10 (StataCorp, College Station, TX, USA), and R v.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
A total of 1750 patients undergoing surgical treatment for renal masses between 1999 and 2009 were identified. Of this cohort, a calculated Nephrometry score based on available preoperative cross-sectional imaging was available for 525 renal masses (30%), all treated between 2007 and 2009. Table 2 summarizes the demographic, clinical, and pathologic variables.
Table 2.
– Demographic, clinical, and pathologic information of all identified patients with Nephrometry score classification on preoperative imaging undergoing surgical resection for a renal mass (n = 525)
| Variable | Patients |
|---|---|
| Demographic and clinical characteristics | |
| Gender, no. (%) | |
| Male | 346 (65.9) |
| Female | 179 (34.1) |
| Age at surgery, yr, median (range) | 60 (25–89) |
| Tumor size, cm, median (range) | 4 (0.7–25) |
| Pathologic characteristics, no. (%) | |
| Tumor histology | |
| Malignant | 453 (86.3) |
| Clear cell RCC | 326 (62.1) |
| Papillary RCC | 77 (14.7) |
| Chromophobe RCC | 30 (5.7) |
| Sarcomatoid RCC | 10 (1.9) |
| Collecting duct RCC | 2 (0.4) |
| Other malignant tumor type | 8 (1.5) |
| Benign | 72 (13.7) |
| Oncocytoma | 40 (70.6) |
| Angiomyolipoma | 18 (3.4) |
| Other benign tumor type | 14 (2.7) |
| Tumor grade, no. (%) | |
| High | 201 (38.3) |
| Low | 242 (46.1) |
| Not specified | 10 (1.9) |
| TNM stage, no. (%) | |
| T1a | 226 (43) |
| T1b | 89 (17) |
| T2 | 44 (8.4) |
| T3a | 53 (10.1) |
| T3b | 37 (7.0) |
| T3c | 1 (0.2) |
| T4 | 3 (0.6) |
| Node status, no. (%) | |
| Nx | 410 (78) |
| N0 | 89 (17) |
| N+ | 26 (5) |
| Presence of metastases, no. (%) | |
| Mx/M0 | 484 (92.1) |
| M1 | 41 (7.8) |
| Anatomic characteristics as captured by Nephrometry, mean (SD; range) | |
| Total Nephrometry score | 8.16 (2.03; 4–12) |
| R attribute | 1.68 (0.81; 1–3) |
| E attribute | 1.73 (0.67; 1–3) |
| N attribute | 2.50 (0.83; 1–3) |
| L attribute | 2.26 (0.82; 1–3) |
RCC = renal cell carcinoma; SD = standard deviation.
Total Nephrometry score and individual anatomic descriptor components significantly differed between high- and low-grade tumors and tumor histology (Table 3). Table 4 describes patient and tumor characteristics associated with malignant or high-grade features on multivariable analysis. Tumor size (R; p < 0.0001), exophycity (E; p = 0.041), and location in relation to the renal poles (L; p = 0.002) were associated with high-grade features.
Table 3.
– Association between tumor anatomic attributes assessed by Nephrometry score, (a) grade, and (b) tumor histology*
| (a) | ||
|---|---|---|
| Grade | Distribution of high- versus low-grade pathology by NS and components | Direction of association (p value) |
| Nephrometry sum | Low grade: mean 7.69; SD 2.1 | Higher NS associated with higher grade (p < 0.0001) |
| High grade: mean 8.93; SD 1.82 | ||
|
| ||
| Radius (diameter) | Low grade (1: 64.9%, 2: 21.1%, 3: 14.1%) | Higher R associated with higher grade (p < 0.0001) |
| High grade (1: 32.3%, 2: 30.9%, 3: 36.8%) | ||
|
| ||
| Exophytic/endophytic properties | Low grade (1: 42.6%, 2: 43.0%, 3: 14.5%) | None (p = 0.076) |
| High grade (1: 38.8%, 2: 52.2%, 3: 9.0%) | ||
|
| ||
| Nearness of the tumor to the collecting system or sinus | Low grade (1: 25.6%, 2: 7.9%, 3: 66.5%) | Higher N associated with higher grade (p = 0.001) |
| High grade (1: 13.4%, 2: 4.5%, 3: 82.1%) | ||
|
| ||
| Anterior or posterior or X | Low grade (A: 51.9%, P: 34.4%, X: 13.7%) | Higher grade associated with X (p = 0.03) |
| High grade (A: 41.3%, P: 36.8%, X: 21.9%) | ||
|
| ||
| Location relative to the polar lines* | Low grade (1: 34.3%, 2: 24.4%, 3: 41.3%) | Higher L associated with higher grade (p < 0.0001) |
| High grade (1: 11.4%, 2: 27.4%, 3: 61.2%) | ||
|
| ||
| Hilar location (abutting main artery or vein) | Low grade (hilar: 16.9%, nonhilar: 83.1%) | Hilar associated with higher grade (p = 0.013) |
| High grade (hilar: 28.9%, nonhilar: 71.1%) | ||
|
| ||
| (b) | ||
|
| ||
| Tumor histology | Distribution of histologic subtypes by NS and components | Ranking of histologic subtypes by NS and components |
|
| ||
| Nephrometry sum | Clear cell: mean 8.35; SD 1.95 | Papillary < benign < chromophobe < clear cell < other p < 0.0001 |
| Papillary: mean 7.29; SD 2.19 | ||
| Chromophobe: mean 8.37; SD 2.06 | ||
| Other: mean 9.03; SD 2.10 | ||
| Benign: mean 7.64; SD 1.77 | ||
|
| ||
| Radius (diameter) | Clear cell (1: 50.9%, 2: 27.9%, 3: 21.2%) | Benign < papillary < clear cell < chromophobe < other p < 0.0001 |
| Papillary (1: 59.7%, 2: 20.8%, 3: 19.5%) | ||
| Chromophobe (1: 60.0%, 2: 13.3%, 3: 26.7%) | ||
| Other (1: 29.4%, 2: 17.7%, 3: 52.9%) | ||
| Benign (1: 74.1%, 2: 20.7%, 3: 5.2%) | ||
|
| ||
| Exophytic/endophytic properties | Clear cell (1: 35.6%, 2: 52.2%, 3: 12.3%) | Other < papillary < chromophobe < clear cell < benign p = 0.030 |
| Papillary (1: 57.1%, 2: 32.5%, 3: 10.4%) | ||
| Chromophobe (1: 40.0%, 2: 46.7%, 3: 13.3%) | ||
| Other (1: 52.9%, 2: 38.2%, 3: 8.8%) | ||
| Benign (1: 34.5%, 2: 46.5%, 3: 19.0%) | ||
|
| ||
| Nearness of the tumor to the collecting system or sinus | Clear cell (1: 18.1%, 2: 6.8%, 3: 75.2%) | Papillary < benign < clear cell < chromophobe < other p = 0.0007 |
| Papillary (1: 36.4%, 2: 7.8%, 3: 55.9%) | ||
| Chromophobe (1: 16.7%, 2: 0%, 3: 83.3%) | ||
| Other (1: 14.7%, 2: 0%, 3: 85.3%) | ||
| Benign (1: 27.6%, 2: 12.1%, 3: 60.3%) | ||
|
| ||
| Anterior or posterior or X | Clear cell (1: 46.5%, 2: 38.5%, 3: 15.1%) | (No relationship) p = 0.61 |
| Papillary (1: 53.3%, 2: 28.6%, 3: 18.2%) | ||
| Chromophobe (1: 46.7%, 2: 30.0%, 3: 23.3%) | ||
| Other (1: 44.1%, 2: 29.4%, 3: 26.5%) | ||
| Benign (1: 46.6%, 2: 37.9%, 3: 15.5%) | ||
|
| ||
| Location relative to the polar lines* | Clear cell (1: 22.7%, 2: 23.6%, 3: 53.7%) | Papillary < benign < chromophobe < clear cell < other p = 0.005 |
| Papillary (1: 36.4%, 2: 31.2%, 3: 32.5%) | ||
| Chromophobe (1: 20.0%, 2: 30.0%, 3: 50.0%) | ||
| Other (1: 11.8%, 2: 23.5%, 3: 64.7%) | ||
| Benign (1: 22.4%, 2: 39.7%, 3: 37.9%) | ||
|
| ||
| Hilar location (abutting main artery or vein) | Clear cell (hilar: 75.8%, nonhilar: 24.2%) | Benign < papillary < chromophobe < clear cell < other p = 0.0004 |
| Papillary (hilar: 87.0%, nonhilar: 13.0%) | ||
| Chromophobe (hilar: 86.7%, nonhilar: 13.3%) | ||
| Other (hilar: 61.8%, nonhilar: 38.2%) | ||
| Benign (hilar: 93.1%, nonhilar: 6.9%) | ||
A = anterior; E = exophytic/endophytic; L = location; N = nearness; NS = nephrometry sum; P = posterior; R = radius; SD = standard deviation.
For each histologic subtype, the ranking was prioritized by the relative contribution of each individual anatomic attribute to overall Nephrometry score. For example, the total N score to the collecting system was statistically lower for papillary tumors than for other histologies, suggesting that papillary tumors less often abut the collecting system. Other refers to sarcomatoid, collecting duct, and other malignant histologic types.
Table 4.
– The p values from likelihood ratio tests of coefficients in simple and multiple logistic regressions evaluating the relationship of demographic/clinical characteristics with (a) malignant histology and (b) tumor grade*
| (a) | ||||
|---|---|---|---|---|
| Covariate | Malignant (1) vs benign (0) | |||
| Unadjusted OR (95% CI) | Unadjusted p value | Full model OR (95% CI) | Full model p value | |
| Gender main effects | 3.23 (1.94–5.38) | <0.001 | 7.38 (0.31–178) | 0.196 |
| Age main effect† | 0.99 (0.97–1.01) | 0.237 | 0.98 (0.92–1.04) | 0.772 |
| Age spline term | Multivariable only | – | 1.03 (0.95–1.11) | – |
| Sex–age interaction‡ | Multivariable only | – | 0.99 (0.89–1.12) | 0.097 |
| Interaction spline term | Multivariable only | – | 0.95 (0.83–1.08) | – |
| R score | – | 0.0003 | – | 0.012 |
| R: 1 | Reference | – | Reference | – |
| R: 2 | 1.71 (0.92–3.17) | – | 1.65 (0.81–3.36) | – |
| R: 3 | 4.86 (1.89–12.52) | – | 4.05 (1.44–11.4) | – |
| E score | 0.339 | – | 0.454 | |
| E: 1 | 1.74 (0.83–3.61) | – | 1.7 (0.68–4.27) | – |
| E: 2 | 1.61 (0.79–3.26) | – | 1.67 (0.74–3.74) | – |
| E: 3 | Reference | – | Reference | – |
| N score | – | 0.0996 | – | 0.312 |
| N: 1 | Reference | – | Reference | – |
| N: 2 or 3 | 1.62 (0.93–2.82) | – | 1.44 (0.71–2.93) | – |
| L score | – | 0.243 | – | 0.130 |
| L: 1 | 1.37 (0.70–2.67) | – | 2.09 (1.01–4.31) | – |
| L: 2 | Reference | – | Reference | – |
| L: 3 | 1.65 (0.92–2.93) | – | 1.49 (0.77–2.88) | – |
| H score | – | 0.007 | – | 0.066 |
| No H | Reference | – | Reference | – |
| H | 2.73 (1.22–6.14) | – | 2.17 (0.9–5.19) | – |
| Covariate | High (1) vs low (0) grade | |||
| Unadjusted OR (95% CI) | Unadjusted p value | Full model OR (95% CI) | Full model p value | |
| Gender main effects | 1.17 (0.78–1.76) | 0.443 | 10.65 (0.88–129) | 0.053 |
| Age main effect† | 1.01 (1.00–1.03) | 0.086 | 1.05 (0.97–1.14) | 0.189 |
| Age spline term | Multivariable only | – | 0.97 (0.89–1.06) | – |
| Sex–age interaction‡ | Multivariable only | – | 0.92 (0.84–1.01) | 0.203 |
| Interaction | Multivariable | – | 1.09 | – |
| spline term | only | (0.98–1.21) | ||
| R score | – | <0.001 | – | <0.001 |
| R: 1 | Reference | – | Reference | – |
| R: 2 | 2.94 (1.84–4.7) | – | 2.26 (1.33–3.83) | – |
| R: 3 | 5.26 (3.19–8.65) | – | 3.89 (2.13–7.13) | – |
| E score | – | 0.074 | – | 0.041 |
| E: 1 | 1.47 (0.78–2.79) | – | 1.24 (0.58–2.66) | – |
| E: 2 | 1.96 (1.05–3.69) | – | 2.00 (1.00–4.01) | – |
| E: 3 | Reference | – | Reference | – |
| N score | – | 0.001 | – | 0.951 |
| N: 1 | Reference | – | Reference | – |
| N: 2 or 3 | 2.22 (1.35–3.65) | – | 0.98 (0.53–1.82) | – |
| L score | – | <0.001 | – | 0.002 |
| L: 1 | 0.30 (0.16–0.54) | – | 0.39 (0.21–0.72) | – |
| L: 2 | Reference | – | Reference | – |
| L: 3 | 1.32 (0.84–2.07) | – | 1.06 (0.63–1.76) | – |
| H score | – | 0.003 | – | 0.583 |
| No H | Reference | – | Reference | – |
| H | 1.99 (1.26–3.13) | – | 1.16 (0.69–1.95) | – |
OR = odds ratio; CI = confidence interval; E = exophytic/endophytic; H = hilar; L = location; N = nearness; NS = nephrometry sum; R = radius; SD = standard deviation.
Although the model coefficients are not reported in this table, they were used to create the nomogram that allows for a visualization of the magnitude of the associations: n = 525 for malignant regression; n = 443* for high-grade regression.
Age was entered into the model after subtracting the minimum age (age minus 25) via a restricted cubic spline with three knots at empirical quantiles. The Nephrometry component scores were entered as categorical dummy variables using the groupings shown in the nomograms. Where two terms were entered into the model for a variable, the p value represents the joint hypothesis test of both parameters. For example, two E components and two age spline terms were entered into the models.
Ten individuals with cancer but missing grade values were excluded from the high-versus low-grade analysis.
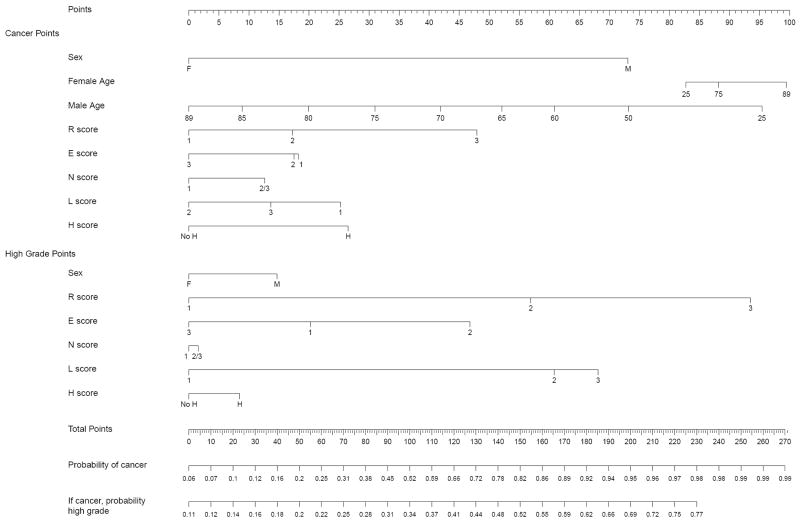
Based on these data, we constructed a predictive nomogram integrating anatomic tumor attributes with patient’s age and gender for preoperative prediction of tumor characteristics. The nomogram combines two independent models. The first model predicts likelihood of a given renal mass proving to be a malignancy upon resection; the second model generates a probability of the tumor being high grade if it is indeed a malignancy. Figure 1 shows the results of the model calibration, which demonstrates that the model is well calibrated. The full nomogram incorporating both the malignant and grade components is presented in Figure 2 and was operationalized for ease during clinical use at www.cancernomograms.com.
Fig. 1.
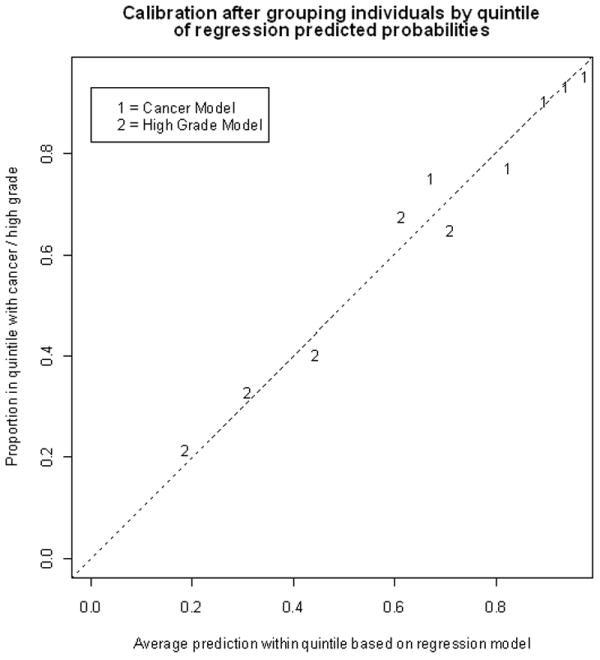
– Calibration after grouping individuals by decile of regression predicted probabilities. Area under the curve (AUC) for cancer model: 0.76; AUC for grade model: 0.73.
In a well calibrated plot, the points would lie on the dashed line. This model is well calibrated. AUC = area under the curve
Fig. 2.
– Nomogram evaluating risks of an enhancing renal mass being malignant and high grade. Total point values are independently calculated for the cancer and the high-grade models and then applied to the corresponding probability scale at the bottom of the figure.
The AUC for the malignant versus benign model was calculated to be 0.76. If the individual Nephrometry score attributes were excluded from the model and only sex and age were considered, the AUC dropped to 0.69 (p = 0.0001 for comparison with final model). Adding tumor size (R) to the model in absence of the other anatomic attributes resulted in an AUC of 0.74 (p = 0.165 for comparison with final model). the AUC for the high-versus low-grade model was 0.73. Nephrometry score significantly contributed to the predictive value of this model because the AUC was 0.52 if only patient age and sex were considered (p < 0.0001 for comparison with final model). The AUC with the inclusion of tumor radius (R) in addition to age and sex improved to 0.68 (p = 0.005 for comparison with final model).
The average AUCs in our out-of-sample cross-validation simulation were 0.68 for the cancer model and 0.69 for the grade model. The 95% range of AUCs was 0.61–0.75 for the cancer model and 0.64–0.74 for the grade model.
4. Discussion
The enhancing renal mass is a heterogeneous clinical entity with varied biologic potential. The ability to match renal mass biology with an appropriate treatment strategy remains an elusive goal of modern urologic oncology [19]. Our results suggest that preoperative radiographic and anatomic attributes of renal masses possess predictive information regarding the mass’s pathologic features. In this study we quantified anatomic complexity using the RENAL Nephrometry score, the first scoring system designed to assess pertinent renal tumor anatomy as it relates to surgical respectability [12]. Alternative systems including preoperative aspects and dimensions used for an anatomical (PADUA) classification and the centrality index (C index) have been reported. The PADUA system quantifies renal mass anatomic features that are similar to those captured by Nephrometry [13], whereas the C index is a calculation that maps the tumor’s relationship to the renal center [14].
The pretreatment assessment of enhancing renal mass pathology has been composed to date primarily of percutaneous biopsy. However, the use of renal biopsy has historically been limited due to risks, sampling error, and the relatively high frequency with which clinically irrelevant data are obtained [6]. The usefulness of percutaneous renal biopsy has been reexamined in select patients [20], and modern series using 18-gauge core biopsies have reported improved accuracy in differentiating benign from malignant histologic types with minimal procedure-related complications [21]. When a malignancy is found on biopsy, the positive predictive value is reported to be >95%. Negative predictive value appears to be >80% with most contemporary series reporting false-negative rates <5% [6,21-23]. Despite the reported accuracy of renal biopsy in determining histologic subtype, most modern series do not assess tumor grade [6], which has significant established prognostic implications for cancer-specific survival [24]. In fact, two contemporary series using modern core biopsy techniques have reported distinction of tumor grading in only 62.7% of patients undergoing biopsy as part of an active surveillance protocol [25]. Underestimation of nuclear grade has been noted in more than half of the patients (55%) undergoing biopsy before surgical resection [26].
At the same time, recent studies suggesting that SRMs grow slowly and have low metastatic potential have led to increased interest in expectant management as an alternative to surgery in elderly patients and those with competing risks of mortality [27]. This has been supported by data suggesting that as many as 20–30% of SRMs <2 cm are benign and that <10–30% are high-grade malignancies [4]. It has been demonstrated that, as tumor size increases, there is a significantly greater probability of malignant versus benign pathology, high-grade versus low-grade disease, and clear cell versus papillary histology [28-30]. Using these data, algorithms have been developed with the aim of predicting the biologic potential of SRMs before intervention. For example, Lane et al constructed a nomogram based on the findings that gender, tumor size, and smoking history were predictive of malignant versus benign disease. However, the concordance index (CI) of this model was a modest 0.64. Furthermore, efforts to differentiate low- from high-grade cancers yielded a CI of 0.56, a model with an accuracy that is only slightly better than a flip of a coin [8]. Using a multi-institutional data set of European patients, Jeldres et al [7] also attempted to develop a tool to predict high grade (Fuhrman grade III–IV) features at nephrectomy, using four covariates: age at diagnosis, gender, tumor size, and symptom classification. Of these factors, only tumor size was significantly associated with high-grade disease on univariate analysis, and their multivariable model to predict high-grade disease was only 58.3% accurate [7].
Given the limitations of tumor size alone to predict pathology and hence biology, efforts to incorporate anatomic features for prediction of renal mass histology have been described as well. For example, Schachter et al reported that exophytic lesions were significantly more likely to be non–clear cell tumors when compared with central lesions (p = 0.003), although no standardized definition of “exophytic” was provided [10]. In 123 patients undergoing laparoscopic partial nephrectomy, Venkatesh et al reported that only 55% of “highly exophytic” tumors were malignant, and of those, nearly all (96%) were low grade [11].
Integration of tumor anatomic attributes with patient age and gender resulted in a predictive model with an AUC of 0.76. In turn, the model for tumor grade resulted in an AUC of 0.73, which appears superior to previously described predictive models [7]. Although direct retrospective comparisons between different cohorts must be interpreted with extreme caution, the predictive ability of this nomogram appears to be similar to the accuracy of contemporary percutaneous biopsy results (approximately 70% accuracy) [25,26]. The grade model appeared to largely retain its accuracy on out-of sample cross-validation. Our initial attempts at integrating preoperative imaging characteristics into a predictive model appear to offer a clinically useful and a robust tool that can help inform critical decision making. Although limited by relatively small sample size and lack of validation with an external data set, the model is the first of its kind to integrate quantifiable tumor anatomic data into a clinical instrument. Using the model, quantifiable probabilities of harboring malignant and high-grade pathology can be objectively compared with competing risks of comorbid medical conditions and the morbidity of treatment itself (Table 5).
Table 5.
– Predicted probability of pathologic outcomes based on anatomic attributes of enhancing renal mass*
| Nephrometry score by component | Probability of malignant lesion, a (%) | Probability of high-grade lesion, b (%) | Probability of a high-grade malignancy, a × b (%) | |
|---|---|---|---|---|
| 50-year-old man with a 8-cm partially exophytic renal mass that crossed the polar line and is abutting the collecting system/hilum | 3 + 2 + 3 + a + 2 + h = 10 ah | 99 | 76 | 75 |
| 80-year-old woman with a 5-cm partially exophytic interpolar mass abutting the hilum | 2 + 2 + 2 + a + 3 + h = 9 ah | 92 | 62 | 57 |
| 50-year-old man with a 2-cm exophytic upper pole renal mass | 1 + 1 + 1 + p + 1 = 4 p | 94 | 17 | 16 |
| 80-year-old man with a 3-cm endophytic mass crossing the polar line | 1 + 3 + 1 + a + 2 = 7 a | 47 | 27 | 13 |
| 50-year-old woman with a 2-cm endophytic interpolar renal mass | 1 + 2 + 1 + a + 2 = 5 a | 54 | 37 | 20 |
When taken together, these data can predict the probability of having a high-grade renal cancer and can then be used to counsel a patient in the setting of competing risks.
5. Conclusions
The prediction of aggressive tumor characteristics to match treatment strategies to tumor biology remains a significant challenge for patients diagnosed with a SRM. Increasing evidence suggests that anatomic features may provide insight into renal tumor biology. We provide a quantitative tool using anatomic features as measured by Nephrometry and demographic features to predict tumor pathology. These data, although promising and novel, await external validation.
Acknowledgments
Funding/Support and role of the sponsor: This publication was supported in part by grant number P30 CA006927 from the National Cancer Institute and by the Department of Defense, Physician Research Training Award (AK). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, or the Department of Defense. Additional funds were provided by Fox Chase Cancer via institutional support of the Kidney Cancer Keystone Program. The role of the funding organization was the management of the data and analysis.
Acknowledgment statement: The authors would like to thank Debra Kister and Michelle Collins for their expertise and support of the Fox Chase Kidney Cancer Database.
Footnotes
Author contributions: Alexander Kutikov had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kutikov, Egleston, Uzzo.
Acquisition of data: Kutikov, Egleston, Uzzo, Canter, Manley, Chen, Viterbo, Greenberg.
Analysis and interpretation of data: Kutikov, Egleston, Uzzo.
Drafting of the manuscript: Kutikov, Smaldone, Egleston, Uzzo, Canter.
Critical revision of the manuscript for important intellectual content: Kutikov, Smaldone, Egleston, Uzzo, Manley, Boorjian, Canter, Simhan, Chen, Viterbo, Greenberg.
Statistical analysis: Egleston.
Obtaining funding: Kitikov, Uzzo.
Administrative, technical, or material support: Uzzo, Greenberg.
Supervision: Kutikov, Uzzo.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/ affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parsons JK, Schoenberg MS, Carter HB. Incidental renal tumors: casting doubt on the efficacy of early intervention. Urology. 2001;57:1013–5. doi: 10.1016/s0090-4295(01)00991-8. [DOI] [PubMed] [Google Scholar]
- 2.Russo P, Jang TL, Pettus JA, et al. Survival rates after resection for localized kidney cancer: 1989 to 2004. Cancer. 2008;113:84–96. doi: 10.1002/cncr.23520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–9. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Frank I, Blute ML, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217–20. doi: 10.1097/01.ju.0000095475.12515.5e. [DOI] [PubMed] [Google Scholar]
- 5.Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–4. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 6.Lane BR, Samplaski MK, Herts BR, et al. Renal mass biopsy—a renaissance? J Urol. 2008;179:20–7. doi: 10.1016/j.juro.2007.08.124. [DOI] [PubMed] [Google Scholar]
- 7.Jeldres C, Sun M, Liberman D. Can renal mass biopsy assessment of tumor grade be safely substituted for by a predictive model? J Urol. 2009;182:2585–9. doi: 10.1016/j.juro.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 8.Lane BR, Babineau D, Kattan MW, et al. A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol. 2007;178:429–34. doi: 10.1016/j.juro.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 9.Weizer AZ, Gilbert SM, Roberts WW, et al. Tailoring technique of laparoscopic partial nephrectomy to tumor characteristics. J Urol. 2008;180:1273–8. doi: 10.1016/j.juro.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 10.Schachter LR, Bach AM, Snyder ME, et al. The impact of tumour location on the histological subtype of renal cortical tumours. BJU Int. 2006;98:63–6. doi: 10.1111/j.1464-410X.2006.06179.x. [DOI] [PubMed] [Google Scholar]
- 11.Venkatesh R, Weld K, Ames CD, et al. Laparoscopic partial nephrectomy for renal masses: effect of tumor location. Urology. 2006;67:1169–74. doi: 10.1016/j.urology.2006.01.089. discussion 1174. [DOI] [PubMed] [Google Scholar]
- 12.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–53. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Ficarra V, Novara G, Secco S, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. 2009;56:786–93. doi: 10.1016/j.eururo.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 14.Simmons MN, Ching CB, Samplaski MK, et al. Kidney tumor location measurement using the C index method. J Urol. 2010;183:1708–13. doi: 10.1016/j.juro.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Green FL. American Cancer Society: AJCC cancer staging manual. 6. New York, NY: Springer-Verlag; 2002. American Joint Committee on Cancer. [Google Scholar]
- 16.Eble J. World Health Organization: Pathology and genetics of tumours of the urinary system and male genital organs. Oxford, UK: IARC Press; 2004. [Google Scholar]
- 17.Harrell FE. Regression modeling strategies. Chapter 2. New York, NY: Springer; 2001. [Google Scholar]
- 18.Kattan MW, Heller G, Brennan MF. A competing-risks nomogram for sarcoma-specific death following local recurrence. Stat Med. 2003;22:3515–25. doi: 10.1002/sim.1574. [DOI] [PubMed] [Google Scholar]
- 19.Uzzo RG. Renal masses—to treat or not to treat? If that is the question are contemporary biomarkers the answer? J Urol. 2008;180:433–4. doi: 10.1016/j.juro.2008.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crispen PL, Blute ML. Do percutaneous renal tumor biopsies at initial presentation affect treatment strategies? Eur Urol. 2009;55:307–9. doi: 10.1016/j.eururo.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 21.Wang R, Wolf JS, Jr, Wood DP, Jr, et al. Accuracy of percutaneous core biopsy in management of small renal masses. Urology. 2009;73:586–90. doi: 10.1016/j.urology.2008.08.519. discussion 590–1. [DOI] [PubMed] [Google Scholar]
- 22.Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281–7. doi: 10.2214/ajr.180.5.1801281. [DOI] [PubMed] [Google Scholar]
- 23.Neuzillet Y, Lechevallier E, Andre M, et al. Accuracy and clinical role of fine needle percutaneous biopsy with computerized tomography guidance of small (less than 4.0 cm) renal masses. J Urol. 2004;171:1802–5. doi: 10.1097/01.ju.0000120147.51090.2b. [DOI] [PubMed] [Google Scholar]
- 24.Tsui KH, Shvarts O, Smith RB, et al. Renal cell carcinoma: prognostic significance of incidentally detected tumors. J Urol. 2000;163:426–30. doi: 10.1016/s0022-5347(05)67892-5. [DOI] [PubMed] [Google Scholar]
- 25.Leveridge M, Shiff D, Chung H, et al. Small renal mass needle core biopsy: outcomes of non-diagnostic percutaneous biopsy and role of repeat biopsy [abstract 821] J Urol. 2010;183:e321. doi: 10.1016/j.eururo.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 26.Blumenfeld AJ, Guru K, Fuchs GJ, et al. Percutaneous biopsy of renal cell carcinoma underestimates nuclear grade. Urology. 2010;76:610–3. doi: 10.1016/j.urology.2009.09.095. [DOI] [PubMed] [Google Scholar]
- 27.Crispen PL, Viterbo R, Boorjian SA, et al. Natural history, growth kinetics, and outcomes of untreated clinically localized renal tumors under active surveillance. Cancer. 2009;115:2844–52. doi: 10.1002/cncr.24338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsivian M, Mouraviev V, Albala DM, et al. Clinical predictors of renal mass pathological features. BJU Int. 2011;107:735–40. doi: 10.1111/j.1464-410X.2010.09629.x. [DOI] [PubMed] [Google Scholar]
- 29.Rothman J, Egleston B, Wong YN, et al. Histopathological characteristics of localized renal cell carcinoma correlate with tumor size: a SEER analysis. J Urol. 2009;181:29–33. doi: 10.1016/j.juro.2008.09.009. discussion 33–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson RH, Hill JR, Babayev Y, et al. Metastatic renal cell carcinoma risk according to tumor size. J Urol. 2009;182:41–5. doi: 10.1016/j.juro.2009.02.128. [DOI] [PMC free article] [PubMed] [Google Scholar]