Abstract
Background
Oncologic outcomes in men with radiation-recurrent prostate cancer (PCa) treated with salvage radical prostatectomy (SRP) are poorly defined.
Objective
To identify predictors of biochemical recurrence (BCR), metastasis, and death following SRP to help select patients who may benefit from SRP.
Design, setting, and participants
This is a retrospective, international, multi-institutional cohort analysis. There was a median follow-up of 4.4 yr following SRP performed on 404 men with radiation-recurrent PCa from 1985 to 2009 in tertiary centers.
Intervention
Open SRP.
Measurements
BCR after SRP was defined as a serum prostate-specific antigen (PSA) ≥0.1 or ≥0.2 ng/ml (depending on the institution). Secondary end points included progression to metastasis and cancer-specific death.
Results and limitations
Median age at SRP was 65 yr of age, and median pre-SRP PSA was 4.5 ng/ml. Following SRP, 195 patients experienced BCR, 64 developed metastases, and 40 died from PCa. At 10 yr after SRP, BCR-free survival, metastasis-free survival, and cancer-specific survival (CSS) probabilities were 37% (95% confidence interval [CI], 31–43), 77% (95% CI, 71–82), and 83% (95% CI, 76–88), respectively. On preoperative multivariable analysis, pre-SRP PSA and Gleason score at postradiation prostate biopsy predicted BCR (p = 0.022; global p < 0.001) and metastasis (p = 0.022; global p < 0.001). On postoperative multivariable analysis, pre-SRP PSA and pathologic Gleason score at SRP predicted BCR (p = 0.014; global p < 0.001) and metastasis (p < 0.001; global p < 0.001). Lymph node involvement (LNI) also predicted metastasis (p = 0.017). The main limitations of this study are its retrospective design and the follow-up period.
Conclusions
In a select group of patients who underwent SRP for radiation-recurrent PCa, freedom from clinical metastasis was observed in >75% of patients 10 yr after surgery. Patients with lower pre-SRP PSA levels and lower postradiation prostate biopsy Gleason score have the highest probability of cure from SRP.
Keywords: Prostate cancer, Radiation therapy, Salvage therapy
1. Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer and the second leading cause of cancer death in men in the United States [1]. Although local therapies with curative intent, such as primary radical prostatectomy (RP) and radiation therapy (RT), may result in durable cancer control in most cases, up to 30% of patients experience biochemical recurrence (BCR) [2–4]. Left untreated, more than a quarter of patients with BCR after RT develop local disease progression at 5 yr [5]. Historically, salvage RP (SRP) for men with biopsy-proven local recurrence after RT has rarely been performed because of concerns regarding lack of efficacy and high morbidity [5,6,7]. However, improvement in surgical experience has led to improved functional outcomes with lower side effects [8]. Furthermore, there is currently no established method of salvage local therapy after RT [9–11].
The efficacy of contemporary SRP and selection of appropriate candidates for SRP remain poorly investigated. Several single-center studies have investigated the efficacy of SRP for radiation-recurrent PCa [6,7,9,12,13], but conclusions from these studies were limited by their small sample size; single-center nature; and not assessing important end points, such as metastasis and cancer-specific death. Moreover, none of these studies was sufficiently powered to identify pre-SRP variables that could identify patients who could benefit from SRP. Thus, we performed a retrospective assessment of oncologic outcomes after SRP in a multicenter series of patients with radiation-recurrent PCa to identify predictors of post-SRP cancer control.
2. Methods
2.1. Study design and population
Seven participating sites provided information for men treated with SRP at their site. This was an institutional review board–approved study conducted according to the US Health Insurance Portability and Accountability Act guidelines. All institutions (Memorial Sloan-Kettering Cancer Center [MSKCC], Mayo Clinic, Netherlands Cancer Institute, San Raffaele Hospital, Katholieke Universiteit [KU] Leuven, University of Sao Paulo, and Vancouver General Hospital) shared agreements before the initiation of the study and provided the necessary clinical data. Before final analysis, all identified anomalies were resolved through regular communication with all sites, and the database was frozen to produce the final data set.
Between 1985 and 2009, 404 patients with radiation-recurrent PCa underwent SRP at one of the seven academic centers (MSKCC: 162; Mayo Clinic: 124; Netherlands Cancer Institute: 46; San Raffaele Hospital: 25; KU Leuven: 20; University of Sao Paulo: 11; Vancouver General Hospital: 16). RT modalities included brachytherapy, external-beam RT (EBRT), or a combination of both (Table 1). All patients had biopsy-proven PCa recurrence before SRP. None of the patients had radiographic evidence of metastatic disease prior to SRP, and none received hormone therapy for recurrent PCa or other salvage therapy before SRP. Pelvic lymph node dissection (PLND) was not performed in 58 patients because of the surgeon’s decision. Dedicated genitourinary pathologists examined all SRP specimens in accordance with the guidelines of the College of American Pathologists [14].
Table 1.
Patient preoperative characteristics*
| Feature | Overall (n = 404) |
|---|---|
| Pre-SRP PSA, ng/ml | |
| Median | 4.5 (2.5–7.4) |
| Range | 0.1–105 |
| RT modality, no. (%) | |
| Brachytherapy, external beam | 11 (3) |
| Brachytherapy, external beam, IMRT | 2 (0) |
| Brachytherapy alone | 76 (19) |
| External beam, 3-DCRT | 5 (1) |
| External beam, IMRT | 5 (1) |
| External beam alone | 253 (63) |
| Unknown | 52 (13) |
| Biopsy Gleason score before SRP, no. (%) | |
| ≤6 | 95 (24) |
| 7 | 120 (30) |
| ≥8 | 80 (20) |
| Unknown/not graded | 109 (27) |
| Clinical stage before SRP, no. (%) | |
| T1 | 86 (21) |
| T2 | 176 (44) |
| T3 | 72 (18) |
| Unknown | 70 (17) |
SRP = salvage radical prostatectomy; PSA = prostate-specific antigen; RT = radiation therapy; IMRT = intensity-modulated RT; 3-DCRT = three-dimensional conformal RT.
Data are given as frequency (percentage) or median (interquartile range).
After SRP, patient follow-up included periodical digital rectal examination and serum prostate-specific antigen (PSA). Imaging studies were performed at the discretion of the attending physician. At both San Raffaele Hospital and Vancouver General Hospital, BCR was defined as a PSA of 0.2 ng/ml and rising; at all other centers, BCR was defined as a PSA of 0.1 ng/ml and rising. No patient received adjuvant therapy before post-SRP BCR. Cause of death was determined by the treating physicians, by chart review corroborated by death certificates, or by death certificates alone. In cases where death certificates were retrieved and reviewed for cause of death, only men who had known recurrence after SRP, had documented metastatic PCa, and had PCa listed in the death certificate were considered to have died of PCa. Perioperative mortality (any death within 30 d of surgery or before discharge) was censored at time of death for PCa survival analyses.
2.2. Statistical methods
BCR was the primary end point of this study; metastasis and cancer-specific survival (CSS) were the secondary end points. The probability of freedom from BCR, metastasis, or death from PCa following SRP was estimated using Kaplan-Meier methods. We created separate pre- and postoperative multivariable Cox proportional hazards models for prediction of BCR and metastasis. Because of the limited number of deaths from PCa, we used univariate Cox models to evaluate predictors of cancer-specific death. The following variables were included as predictors in the preoperative model: log of PSA before SRP, biopsy Gleason score before SRP (≤6, 7, ≥8, or unknown/not graded), and clinical stage before SRP (T1, T2, T3, or unknown). The following variables were included as predictors in the postoperative model: log of PSA before SRP, pathologic Gleason score at the time of SRP (≤6, 7, ≥8, or unknown/not graded), extraprostatic extension (EPE), seminal vesicle invasion (SVI), and lymph node involvement (LNI) (negative/no PLND or positive).
3. Results
The 404 patients had a median age of 65 yr of age at the time of SRP (interquartile range [IQR]: 60–69), with a median pre-SRP PSA of 4.5 ng/ml (IQR: 2.5–7.4). Overall, the median time interval between RT and SRP was 41 mo (IQR: 27–58). Approximately half of the patients had a post-RT prostate biopsy Gleason score ≥7, and 25% were not graded because of RT treatment effect (Table 1). Clinical stage T3 cancer was present in 72 patients (18%) at the time of recurrence before SRP.
In analyzing SRP specimens, 96 patients (24%) had a Gleason score ≥8, 181 patients (45%) had EPE, 120 patients (30%) had SVI, 65 patients (16%) had LNI, and 99 patients (25%) had positive surgical margins (PSM; Table 2). The number of patients with a Gleason score ≥7 was 22% higher at SRP pathology than at biopsy before SRP (50% and 61%, respectively).
Table 2.
Patients’ pathologic features*
| Feature | n = 404 |
|---|---|
| Pathologic Gleason score, no. (%) | |
| ≤6 | 55 (14) |
| 7 | 150 (37) |
| ≥8 | 96 (24) |
| Unknown/not graded | 103 (25) |
| Surgical margin status, no. (%) | |
| Negative | 301 (75) |
| Positive | 99 (25) |
| Unknown | 4 (1) |
| EPE, no. (%) | |
| No | 213 (53) |
| Yes | 181 (45) |
| Unknown | 10 (2) |
| SVI, no. (%) | |
| No | 275 (68) |
| Yes | 120 (30) |
| Unknown | 9 (2) |
| LNI, no. (%) | |
| Negative/no PLND** | 337 (83) |
| Positive | 65 (16) |
EPE - extraprostatic extension; SVI = seminal vesical invasion; LNI = lymph node involvement; PLND = pelvic lymph node dissection.
Data are given as frequency (percentage).
PLND was not performed in 58 patients.
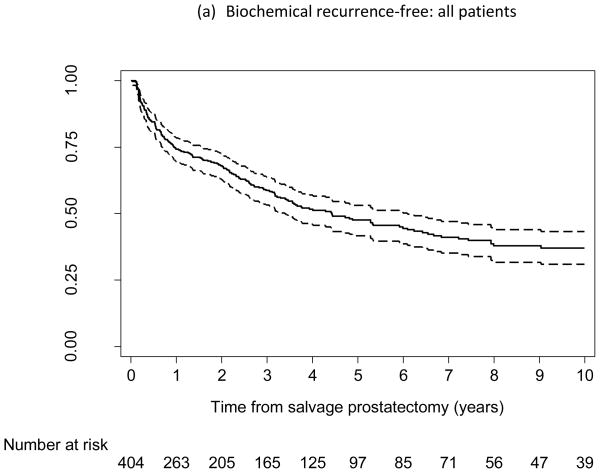
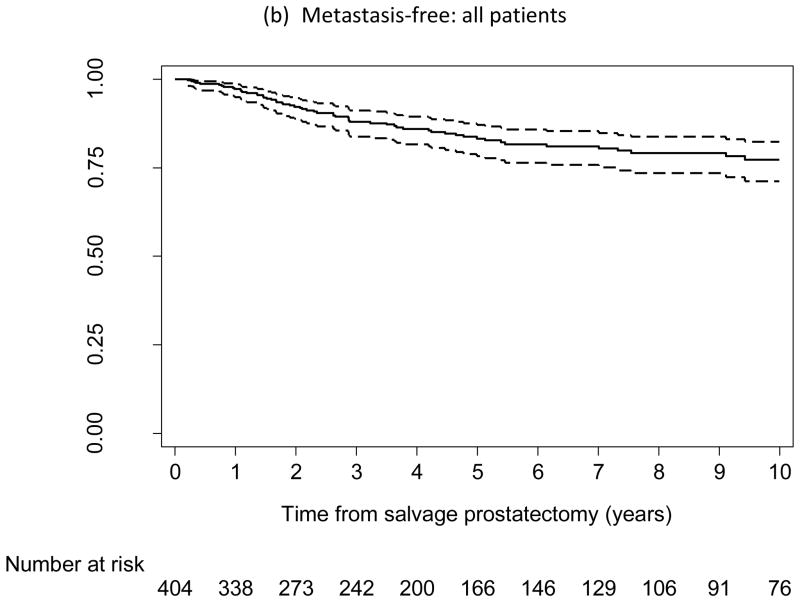
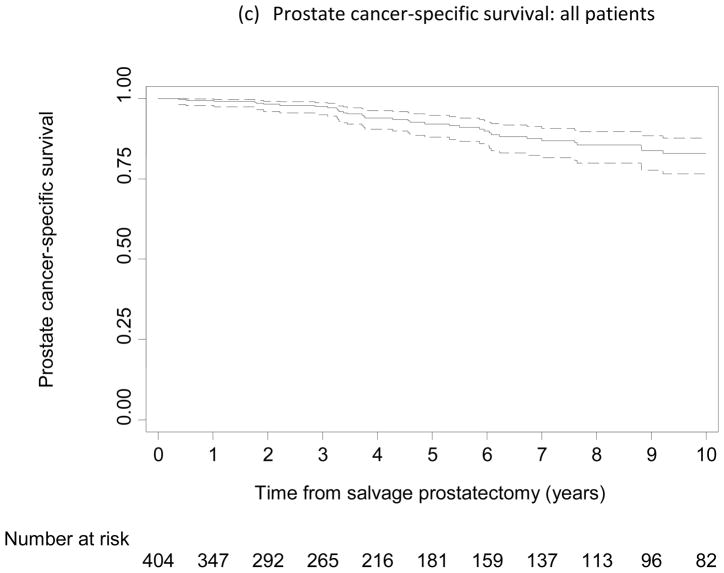
Of the 404 patients, 195 experienced BCR, 64 developed metastasis, and 40 died from PCa. Figure 1a–c shows the probability of being free from BCR, metastasis, and cancer-specific death, respectively. At 5 yr after SRP, the probabilities of being free from BCR, metastasis, and cancer-specific death were 48% (95% confidence interval [CI], 42–53), 83% (95% CI, 78–87), and 92% (95% CI, 88–95), respectively; at 10 yr, the probabilities were 37% (95% CI, 31–43), 77% (95% CI, 71–82), and 83% (95% CI, 76–88), respectively.
Fig. 1.
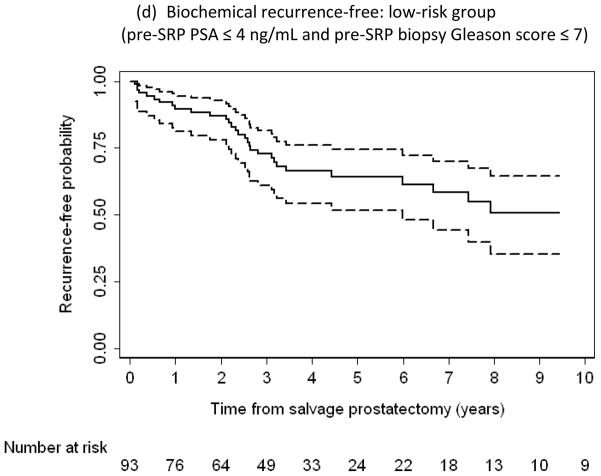
Kaplan-Meier curves reflecting proportion of patients free from (a, d) biochemical recurrence, (b) metastasis, and (c) prostate cancer–specific death following salvage radical prostatectomy (SRP). (d) Low-risk subpopulation (n = 93; pre-SRP prostate-specific antigen ≤4 ng/ml and pre-SRP biopsy Gleason score ≤7). Dashed lines indicate 95% confidence intervals.
On preoperative multivariable analyses, pre-SRP PSA (hazard ratio [HR]: 1.19 per log ng/ml; 95% CI, 1.03–1.39; p = 0.022) and biopsy Gleason score (HR: 2.03 for 7 vs ≤6; 95% CI, 1.28–3.22; HR: 3.22 for ≥8 vs ≤6; 95% CI, 2.02–5.12; global p < 0.001) were significantly associated with BCR (Table 3). Pre-SRP PSA (HR: 1.37 per log ng/ml; 95% CI, 1.05–1.79; p = 0.022) and biopsy Gleason score (HR: 2.77 for 7 vs ≤6; 95% CI, 0.97–7.94; HR: 6.88 for ≥8 vs ≤6; 95% CI, 2.57–18.40; global p < 0.001) were also significant predictors for metastases.
Table 3.
Multivariable Cox proportional hazards regression analyses to evaluate predictors of biochemical recurrence and metastasis following salvage radical prostatectomy
| Feature | Outcome: BCR | Outcome: Metastases | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Preoperative model (n = 376) | ||||||
| Log PSA before SRP, ng/ml | 1.19 | 1.03–1.39 | 0.022 | 1.37 | 1.05–1.79 | 0.022 |
| Biopsy Gleason score before SRP | – | – | <0.001 | – | – | <0.001 |
| ≤6 | Reference | Reference | – | Reference | Reference | – |
| 7 | 2.03 | 1.28–3.22 | – | 2.77 | 0.97–7.94 | – |
| ≥8 | 3.22 | 2.02–5.12 | – | 6.88 | 2.57–18.40 | – |
| Unknown/not graded | 1.94 | 1.24–3.05 | – | 3.31 | 1.24–8.84 | – |
| Clinical stage before SRP | – | – | 0.7 | – | – | 0.14 |
| T1 | Reference | Reference | – | Reference | Reference | – |
| T2 | 1.18 | 0.78–1.78 | – | 2.49 | 0.88–7.10 | – |
| T3 | 1.14 | 0.70–1.87 | – | 3.41 | 1.13–10.30 | – |
| Unknown | 0.92 | 0.53–1.60 | – | 1.67 | 0.44–6.29 | – |
|
| ||||||
| Postoperative model (n = 372) | ||||||
| Log PSA before SRP, ng/ml | 1.21 | 1.04–1.40 | 0.014 | 1.64 | 1.24–2.18 | <0.001 |
| Pathologic Gleason score at SRP | – | – | <0.001 | – | – | <0.001 |
| ≤6 | Reference | Reference | – | Reference | Reference | – |
| 7 | 1.48 | 0.89–2.48 | – | 0.68 | 0.27–1.76 | – |
| ≥8 | 2.64 | 1.55–4.50 | – | 2.70 | 1.11–6.56 | – |
| Unknown/not graded | 1.47 | 0.85–2.51 | – | 0.95 | 0.37–2.44 | – |
| EPE | 1.06 | 0.76–1.47 | 0.7 | 1.53 | 0.84–2.79 | 0.17 |
| SVI | 1.26 | 0.89–1.79 | 0.19 | 1.18 | 0.65–2.14 | 0.6 |
| LNI | 1.10 | 0.75–1.61 | 0.6 | 2.04 | 1.14–3.67 | 0.017 |
BCR = biochemical recurrence; HR = hazard ratio; CI = confidence interval; PSA = prostate-specific antigen; SRP = salvage radical prostatectomy; EPE = extraprostatic extension; SVI = seminal vesical invasion; LNI = lymph node involvement.
In a postoperative multivariable prognostic model that included pre- and postoperative factors, pre-SRP PSA and SRP specimen Gleason score predicted BCR (p = 0.014 and global p < 0.001) and metastases (p < 0.001 and global p < 0.001; Table 3). LNI also predicted metastases (p = 0.017).
Table 4 shows univariate Cox proportional hazards regression analyses to evaluate predictors of death from PCa. Higher pre-SRP PSA, clinical stage, biopsy and pathologic Gleason score, and the presence of SVI were all significantly associated with a higher risk of dying from PCa after SRP.
Table 4.
Univariate Cox proportional hazards regression analyses to evaluate predictors of death from prostate cancer following salvage radical prostatectomy
| Feature | Outcome: Death from PCa | ||
|---|---|---|---|
| HR | 95% CI | p value | |
| Log PSA before SRP, ng/m | 1.85 | 1.32–2.60 | <0.001 |
|
| |||
| Biopsy Gleason score before SRP | |||
| ≤6 | Reference | Reference | 0.04 |
| 7 | 4.14 | 1.12–15.40 | – |
| ≥8 | 8.09 | 2.25–29.10 | – |
| Unknown/not graded | 4.03 | 1.16–14.00 | – |
|
| |||
| Clinical stage before SRP | |||
| T1 | Reference | Reference | 0.03 |
| T2 | 5.81 | 0.78–43.40 | – |
| T3 | 10.60 | 1.39–80.60 | – |
| Unknown | 1.74 | 0.16–19.30 | – |
|
| |||
| Pathology Gleason score at SRP | |||
| ≤6 | Reference | Reference | 0.003 |
| 7 | 0.68 | 0.20–2.22 | – |
| ≥8 | 3.43 | 1.22–9.64 | – |
| Unknown/not graded | 1.87 | 0.67–5.20 | – |
|
| |||
| EPE | 1.67 | 0.85–3.28 | 0.14 |
|
| |||
| SVI | 2.22 | 1.17–4.21 | 0.015 |
|
| |||
| LNI | 1.30 | 0.60–2.84 | 0.5 |
|
| |||
| PSM | 1.80 | 0.96–3.37 | 0.068 |
PCa = prostate cancer; PSA = prostate-specific antigen; SRP = salvage radical prostatectomy; CI = confidence interval; EPE = extraprostatic extension; SVI = seminal vesical invasion; LNI = lymph node involvement; PSM = positive surgical margins.
Based on these multivariable analyses, we identified a more favorable risk group of 93 men who had a pre-SRP prostate biopsy Gleason score ≤7 and pre-SRP PSA ≤4 ng/ml. Of this group, which comprised approximately 30% of our cohort, 52 men experienced BCR, 3 developed metastases, and none died from PCa; the BCR-free probability in these men was 64% (95% CI, 52–74) at 5 yr and 51% (95% CI, 35–64) at 10 yr (Fig. 1d).
4. Discussion
A critical concern in the management of men with rising PSA levels after RT for PCa is determining whether a rising PSA represents recurrence and whether this represents local and/or distant disease. If the recurrence is deemed localized, there is still the opportunity for cure with salvage therapy. SRP represents one such treatment approach. Stephenson et al have previously shown in a large study that one-third of patients were free of disease progression 6 yr after salvage RT, suggesting that recurrent PCa may be affected by a local salvage treatment [15].
Although several features have been associated with a higher likelihood of systemic rather than local disease, including a rapidly rising post-treatment PSA level, short PSA doubling time (DT), poorly differentiated cancer (Gleason score 8–10), and a short disease-free interval after RT, no individual factor is definitively associated with metastatic progression nor eliminates the possible benefit of local salvage therapy [16,17].
Several previous studies have demonstrated the important prognostic variables and established useful predictive tools to improve patient selection and more favorable outcomes associated with salvage RT in men with PCa recurrence after RP [15,18]. Conversely, despite the substantial number of patients who experience PCa recurrence after RT [19], there has been a lack of large studies reporting on post-SRP outcomes and specifically focusing on potentially useful prognostic tools. Moreover, several well-designed trials have addressed the setting of primary RT concerning dose and/or its combination with androgen-deprivation therapy (ADT) [4,20,21], but very few have provided information on selecting patients to receive local salvage therapy for PCa recurrence after RT.
The widespread use of ADT in this setting may be the result of the concern of high morbidity related to SRP, even though consistent evidence shows that ADT cannot be considered a curative treatment for patients with locally recurrent PCa [22]. Surgical complications are certainly a matter of concern; however, studies have suggested that SRP is a safe procedure and shown acceptable functional outcomes [8,23,24]. A recent study with 55 patients has demonstrated a low perioperative complication rate of <10%, with only two cases of rectal injury (3.6%). Moreover, 80% of patients were continent after 1 yr of SRP, and 40% of patients with normal erectile function prior to surgery were able to have sexual intercourse with the use of oral medication [25].
Before our current large, multi-institutional study, reports with small cohorts had already suggested meaningful oncologic outcomes with SRP. Paparel et al found that more than half of the patients who underwent SRP were still free of BCR after 5 yr [7]. Pisters et al showed the superiority of cancer control with SRP when compared to salvage cryotherapy [9]. Sanderson et al also reported good results after SRP and analyzed quality of life (QoL), which showed improvement with artificial urinary sphincter and inflatable penile prosthesis devices when needed [13].
Predictors of oncologic outcomes with the purpose of better selecting candidates for additional local therapy after RT failures have been rarely reported. In a study with 106 patients, post-RT biopsy Gleason score was significantly associated with BCR but not pre-SRP PSA, possibly because of the small sample size [7]. In another study, Heidenreich et al found that pre-SRP biopsy Gleason score, <50% positive biopsy cores, PSA DT >12 mo, and low-dose brachytherapy were significant predictors of organ-confined disease with negative surgical margins [25].
Our data indicate that the most favorable group for undergoing SRP is men with a PSA <4 ng/ml and a postradiation prostate biopsy Gleason score ≤7. Our population had a mean pre-SRP PSA level of 4.5 ng/ml, which indicates that either earlier (lower PSA level) evaluation for recurrence and/or the development of new methods to detect local recurrence are needed to improve oncologic outcomes after SRP.
Patient selection bias was a major limitation of this study and certainly affected our results, because tumor characteristics would have significantly worsened the oncologic outcomes in a patient population with PCa with more aggressive features. In this scenario, more patients would have had disease recurrence despite SRP, leading to a conclusion of a deficient cancer control.
Furthermore, in our current study, we could not ascertain whether SRP was in fact capable of affecting the natural history of the subgroup of patients with low-risk disease, which could never have developed clinical metastasis, considering the biology of PCa. The lack of a control group is the main reason for this important limitation.
For patients in whom primary RT failed, in the select group of patients who did undergo SRP, freedom from clinical metastasis was observed in >75% of patients 10 yr after surgery. We identified preoperative predictors of outcome that might be used to better select men for SRP. Serum PSA level before SRP and prostate biopsy Gleason score were significant predictors of BCR and metastases following SRP.
5. Conclusions
We demonstrated that a local salvage treatment such as SRP can lead to a considerable BCR-free and metastasis-free survival. With pre- and postoperative predictive models, we identified several features associated with BCR and metastasis after SRP. These improved selection criteria may help better stratify patients who would most benefit from SRP, thereby sparing them from ADT and potentially enhancing their QoL. The current study presents tools for estimating the probability of oncologic response to SRP to better assist both physicians and their patients with treatment decisions.
Acknowledgments
Funding/Support and role of the sponsor: The Sidney Kimmel Center for Prostate and Urologic Cancers at MSKCC and David H. Koch through the Prostate Cancer Foundation to MSKCC provided support for this study. Dr Chade was an MSKCC research fellow in urologic oncology supported by CAPES. Dr Shariat was an MSKCC research fellow in urologic oncology supported by NIH T32-CA82088.
Footnotes
Author contributions: Daher C. Chade had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chade, Shariat.
Acquisition of data: Chade, Shariat, Karnes, Blute, Briganti, van derPoel, Van Poppel, Joniau, Godoy, Hurtado-Coll, Gleave, Dall’Oglio, Srougi.
Analysis and interpretation of data: Chade, Shariat, Cronin, Savage, Montorsi, Scardino, Eastham.
Drafting of the manuscript: Chade, Shariat.
Critical revision of the manuscript for important intellectual content: Montorsi, Scardino, Eastham.
Statistical analysis: Cronin, Savage.
Obtaining funding: Chade, Shariat.
Administrative, technical, or material support: Chade, Shariat.
Supervision: Scardino, Eastham.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zelefsky MJ, Eastham JA, Cronin AM, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010;28:1508–13. doi: 10.1200/JCO.2009.22.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289–95. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 5.Lee WR, Hanks GE, Hanlon A. Increasing prostate-specific antigen profile following definitive radiation therapy for localized prostate cancer: clinical observations. J Clin Oncol. 1997;15:230–8. doi: 10.1200/JCO.1997.15.1.230. [DOI] [PubMed] [Google Scholar]
- 6.Leibovici D, Spiess PE, Heller L, Rodriguez-Bigas M, Chang G, Pisters LL. Salvage surgery for locally recurrent prostate cancer after radiation therapy: tricks of the trade. Urol Oncol. 2008;26:9–16. doi: 10.1016/j.urolonc.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Paparel P, Cronin AM, Savage C, Scardino PT, Eastham JA. Oncologic outcome and patterns of recurrence after salvage radical prostatectomy. Eur Urol. 2009;55:404–10. doi: 10.1016/j.eururo.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Gotto GT, Yunis LH, Vora K, Eastham JA, Scardino PT, Rabbani F. Impact of prior prostate radiation on complications after radical prostatectomy. J Urol. 2010;184:136–42. doi: 10.1016/j.juro.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 9.Pisters LL, Leibovici D, Blute M, et al. Locally recurrent prostate cancer after initial radiation therapy: a comparison of salvage radical prostatectomy versus cryotherapy. J Urol. 2009;182:517–25. doi: 10.1016/j.juro.2009.04.006. discussion 525–7. [DOI] [PubMed] [Google Scholar]
- 10.Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–81. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh PC, DeWeese TL, Eisenberger MA. A structured debate: immediate versus deferred androgen suppression in prostate cancer-evidence for deferred treatment. J Urol. 2001;166:508–15. doi: 10.1016/s0022-5347(05)65972-1. discussion 515–6. [DOI] [PubMed] [Google Scholar]
- 12.Bianco FJ, Jr, Scardino PT, Stephenson AJ, Diblasio CJ, Fearn PA, Eastham JA. Long-term oncologic results of salvage radical prostatectomy for locally recurrent prostate cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:448–53. doi: 10.1016/j.ijrobp.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 13.Sanderson KM, Penson DF, Cai J, et al. Salvage radical prostatectomy: quality of life outcomes and long-term oncological control of radiorecurrent prostate cancer. J Urol. 2006;176:2025–31. doi: 10.1016/j.juro.2006.07.075. discussion 2031–2. [DOI] [PubMed] [Google Scholar]
- 14.Henson DE, Hutter RV, Farrow G. Practice protocol for the examination of specimens removed from patients with carcinoma of the prostate gland. A publication of the Cancer Committee, College of American Pathologists. Task Force on the Examination of Specimens Removed from Patients with Prostate Cancer. Arch Pathol Lab Med. 1994;118:779–83. [PubMed] [Google Scholar]
- 15.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues NA, Chen MH, Catalona WJ, Roehl KA, Richie JP, D’Amico AV. Predictors of mortality after androgen-deprivation therapy in patients with rapidly rising prostate-specific antigen levels after local therapy for prostate cancer. Cancer. 2006;107:514–20. doi: 10.1002/cncr.22018. [DOI] [PubMed] [Google Scholar]
- 17.Slovin SF, Wilton AS, Heller G, Scher HI. Time to detectable metastatic disease in patients with rising prostate-specific antigen values following surgery or radiation therapy. Clin Cancer Res. 2005;11:8669–73. doi: 10.1158/1078-0432.CCR-05-1668. [DOI] [PubMed] [Google Scholar]
- 18.Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325–32. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 19.Souhami L, Bae K, Pilepich M, Sandler H. Impact of the duration of adjuvant hormonal therapy in patients with locally advanced prostate cancer treated with radiotherapy: a secondary analysis of RTOG 85-31. J Clin Oncol. 2009;27:2137–43. doi: 10.1200/JCO.2008.17.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292:821–7. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 21.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294:1233–9. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 22.Cooperberg MR, Vickers AJ, Broering JM, Carroll PR. Comparative risk-adjusted mortality outcomes after primary surgery, radiotherapy, or androgen-deprivation therapy for localized prostate cancer. Cancer. 2010;22:5226–34. doi: 10.1002/cncr.25456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darras J, Joniau S, Van Poppel H. Salvage radical prostatectomy for radiorecurrent prostate cancer: indications and results. Eur J Surg Oncol. 2006;32:964–9. doi: 10.1016/j.ejso.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Stephenson AJ, Scardino PT, Bianco FJ, Jr, DiBlasio CJ, Fearn PA, Eastham JA. Morbidity and functional outcomes of salvage radical prostatectomy for locally recurrent prostate cancer after radiation therapy. J Urol. 2004;172:2239–43. doi: 10.1097/01.ju.0000140960.63108.39. [DOI] [PubMed] [Google Scholar]
- 25.Heidenreich A, Richter S, Thuer D, Pfister D. Prognostic parameters, complications, and oncologic and functional outcome of salvage radical prostatectomy for locally recurrent prostate cancer after 21st-century radiotherapy. Eur Urol. 2010;57:437–43. doi: 10.1016/j.eururo.2009.02.041. [DOI] [PubMed] [Google Scholar]