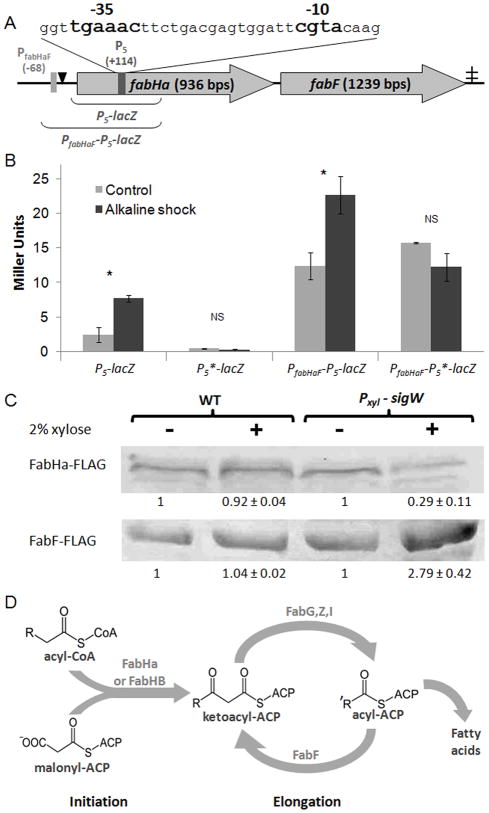
Figure 1.
A σW dependent promoter (P5) in fabHa.
A. Schematic map of P5, PfabHaF, FabHa, FabF, and the associated FapR binding site (black triangle). The -35 and -10 elements of P5 are in bold. The locations of the P5 and PfabHaF transcriptional start sites (+114 and −68 respectively) are described relative to the first base of the FabHa translation initiation codon. The ‡ indicates a putative terminator. The DNA regions included in the PfabHaF-P5-lacZ and P5-lacZ fusions are also illustrated.
B. The β-galactosidase activity of the P5-lacZ (HB13069), P5*-lacZ (HB13080), PfabHaF-P5-lacZ (HB13001), and PfabHaF-P5*-lacZ (HB13082) strains grown to mid-log phase in LB with and without alkaline shock. This experiment was performed in biological triplicate and repeated at least three times. Bars represent mean values with error bars indicating standard error. Student’s t tests were performed, and a statistically significant difference (P value ≥ 0.05) between the control and alkaline shocked cells is denoted as * above the bar graph while a non-significant difference is denoted as NS.
C. Detection of FLAG-tagged FabHa and FabF by Western blot with anti-FLAG antibodies in the strains fabHa-FLAG (HB13054), fabF-FLAG (HB13056), fabHa-FLAG Pxyl-sigW (HB13058), and fabF-FLAG Pxyl-sigW (HB13060) with and without xylose treatment. This experiment was performed in biological triplicates and repeated at least three times. The numbers below each band represent the average intensity of that band (± standard error) relative to the non-xylose treated control for that strain. Using Student’s t-tests, a statistically significant difference (P value ≤ 0.05) between the control and xylose treated cells was found in strains containing the Pxyl-sigW construct but not in strains lacking this construct.
D. Overview of FA biosynthesis. Chain initiation requires either FabHa or FabHb. FabHb has a greater ability to accept straight chain precursors than FabHa. Increased abundance of the FabF elongation enzyme can increase the chain length of the resulting FA (Choi et al., 2000; Schujman and de Mendoza, 2008; and this work).