Figure 7.
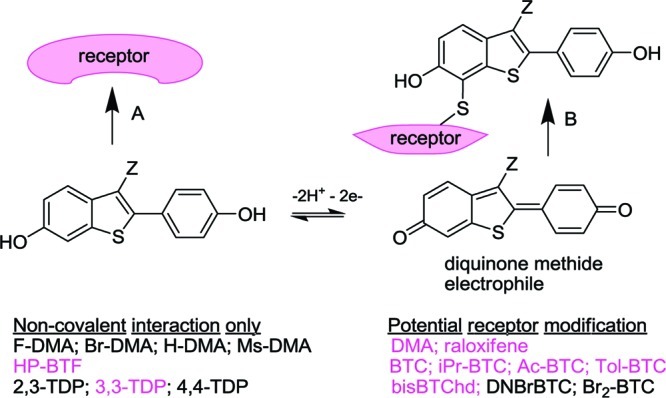
The BTC moiety is readily oxidized to a diquinone methide that acts as a Michael acceptor toward cysteine. The receptor mediating the neuroprotective activity of BT-SERMs could potentially be activated by Michael addition (B) or alternatively by simple noncovalent binding (A). Column 1 lists study compounds including BT-SERMs that cannot undergo bioactivation to diquinone methides. Column 2 lists redox reactive compounds able to form a diquinone methide. Study compounds shown in colored text were observed to deliver GPR30-dependent neuroprotection. The lack of correlation of activity with potential for bioactivation supports a noncovalent receptor binding mode.
