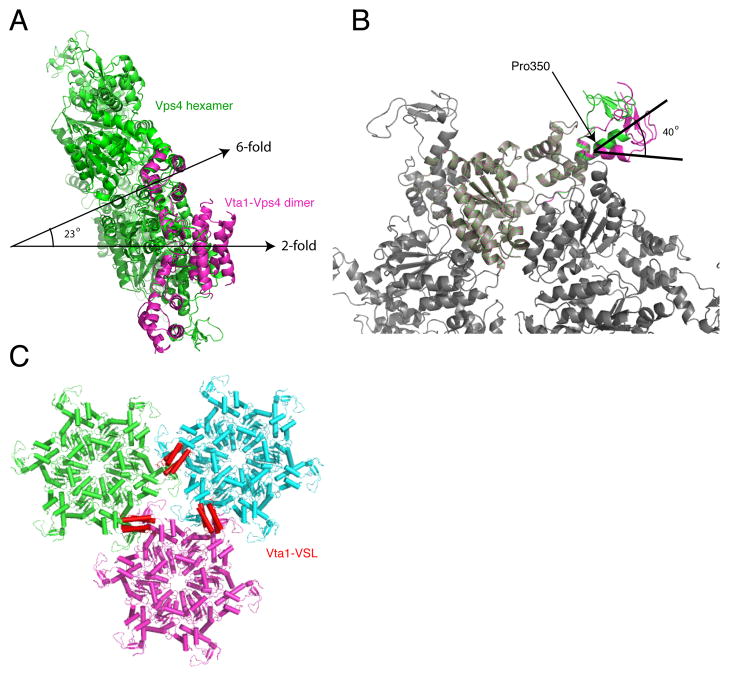
Fig. 7.
A model for cross-linking of Vps4 hexamers by the Vta1-VSL domain. A. Following superposition of the Vps4 SAB domain on one subunit of the Vps4 hexamer (green), the two-fold axis of the Vta1-VLS:Vps4-SAB complex (magenta) is within 23° of the six-fold axis of the hexamer. Part of the second Vps4-SAB overlaps with another subunit in the hexamer. B. Model for a putative unkinked conformation of α8. This model rotates the Vps4 β-domain and Vta1 by 40°, eliminates the steric overlap between the second SAB fragment and the hexamer, and is compatible with continuous hexagonal lattice packing. C. Hypothetical p6 lattice arrangement of Vta1-VSL (red) cross-linked Vps4 lower ring hexamers. For illustration purposes, a model of the human VPS4B hexamer is used instead of yeast, as the longer β6- β7 loop in human makes the orientation of the β domain easier to visualize.